Hydrogen
Hydrogen is the simplest of all chemical elements. It is a colorless, odorless, tasteless gas that burns in air to produce water. It has one of the lowest boiling points, −252.9°C (−423.2°F), and freezing points, −259.3°C (−434.7°F), of all elements.
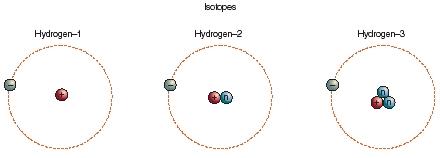
An atom of hydrogen contains one proton and one electron, making it the simplest atom that can be constructed. Because of the one proton in its nucleus, hydrogen is assigned an atomic number of 1. A total of three isotopes of hydrogen exist. Isotopes are forms of an element with the same atomic number but different atomic masses. Protium and deuterium are both stable isotopes, but tritium is radioactive.
Hydrogen is the first element in the periodic table. Its box is situated at the top of Group 1 in the periodic table, but it is not generally considered a member of the alkali family, the other elements that make up Group 1. Its chemical properties are unique among the elements, and it is usually considered to be in a family of its own.
The Hydrogen Economy
Some social scientists have called the last century of human history the Fossil Age. That term comes from the fact that humans have relied so heavily on the fossil fuels—coal, oil, and natural gas—for the energy we need to run our societies. What happens when the fossil fuels are exhausted? Where will humans turn for a new supply of energy?
A new generation of scientists is suggesting the use of hydrogen as a future energy source. Hydrogen burns in air or oxygen with a very hot flame that can be used to generate steam, electricity, and other forms of energy. The only product of that reaction is water, a harmless substance that can be released to the environment without danger. In addition, enormous amounts of hydrogen are available from water. Electrolysis can be used to obtain hydrogen from the world's lakes and oceans.
An economy based on hydrogen rather than the fossil fuels faces some serious problems, however. First, hydrogen is a difficult gas with which to work. It catches fire easily and, under certain circumstances, does so explosively. Also, the cost of producing hydrogen by electrolysis is currently much too high to make the gas a useful fuel for everyday purposes.
Of course, once the fossil fuels are no longer available, humans may have no choice but to solve these problems in order to remain a high-energy-use civilization.
History
Hydrogen was discovered in 1766 by English chemist and physicist Henry Cavendish (1731–1810). It was named by French chemist Antoine-Laurent Lavoisier (1743–1794) from the Greek words for "water-former." Early research on hydrogen was instrumental in revealing the true nature of oxidation (burning) and, therefore, was an important first step in the birth of modern chemistry.
Abundance
Hydrogen is by far the most abundant element in the universe. It makes up about 93 percent of all atoms in the universe and about three-quarters of the total mass of the universe. Hydrogen occurs both within stars and in the interstellar space (the space between stars). Within stars, hydrogen is consumed in nuclear reactions by which stars generate their energy.
Hydrogen is much less common as an element on Earth. Its density is so low that it long ago escaped from Earth's gravitational attraction. Hydrogen does occur on Earth in a number of compounds, however, most prominently in water. Water is the most abundant compound on Earth's surface.
Hydrogen also occurs in nearly all organic compounds and constitutes about 61 percent of all the atoms found in the human body. Chemists now believe that hydrogen forms more compounds than any other element, including carbon.
The Isotopes of Hydrogen
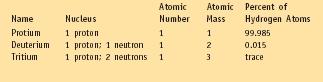
| Atomic | Atomic | Percent of | ||
| Name | Nucleus | Number | Mass | Hydrogen Atoms |
| Protium | 1 proton | 1 | 1 | 99.985 |
| Deuterium | 1 proton; 1 neutron | 1 | 2 | 0.015 |
| Tritium | 1 proton; 2 neutrons | 3 | trace |
Properties and uses
Hydrogen is a relatively inactive element at room temperature, but it becomes much more active at higher temperatures. For example, it burns in air or pure oxygen with a pale blue, almost invisible flame. It can also be made to react with most elements, both metals and nonmetals. When combined with metals, the compounds formed are called hydrides. Some familiar compounds of hydrogen with nonmetals include ammonia (NH 3 ), hydrogen sulfide (H 2 S), hydrogen chloride (or hydrochloric acid, HCl), hydrogen fluoride (or hydrofluoric acid, H 2 F 2 ), and water (H 2 O).
The largest single use of hydrogen is in the production of ammonia. Ammonia, in turn, is used in the production of fertilizers and as a fertilizer itself. It is also a raw material for the production of explosives. Large amounts of hydrogen are also employed in hydrogenation, the process by which hydrogen is reacted with liquid oils to convert them to solid fats. Hydrogen is used in the production of other commercially important chemicals as well, most prominently, hydrogen chloride. Finally, hydrogen acts as a reducing agent in many industrial processes. A reducing agent is a substance that reacts with a metallic ore to convert the ore into a pure metal.
[ See also Periodic table ]
=) ..