Atoms - How it works
Why Study Atoms?
Many accounts of the atom begin with a history of the growth in scientists' understanding of its structure, but here we will take the opposite approach, first discussing the atom in terms of what physicists and chemists today understand. Only then will we examine the many challenges scientists faced in developing the current atomic model: false starts, wrong theories, right roads not taken, incomplete models. In addition, we will explore the many insights added along the way as, piece by piece, the evidence concerning atomic behavior began to accumulate.
People who are not scientifically trained tend to associate studies of the atom with physics, not chemistry. While it is true that physicists study atomic structure, and that much of what scientists know today about atoms comes from the work of physicists, atomic studies are even more integral to chemistry than to physics. At heart, chemistry is about the interaction of different atomic and molecular structures: their properties, their reactions, and the ways in which they bond.
WHAT THE ATOM MEANS TO CHEMISTRY.
Just as a writer in English works with the 26 letters of the alphabet, a chemist works with the 100-plus known elements, the fundamental and indivisible substances of all matter. And what differentiates the elements, ultimately, from one another is not their color or texture, or even the phase of matter—solid, gas, or liquid—in which they are normally found. Rather, the defining characteristic of an element is the atom that forms its basic structure.
The number of protons in an atom is the critical factor in differentiating between elements, while the number of neutrons alongside the protons in the nucleus serves to distinguish one isotope from another. However, as important as elements and even isotopes are to the work of a chemist, the components of the atom's nucleus have little direct bearing on the atomic activity that brings about chemical reactions and chemical bonding. All the chemical "work" of an atom is done by particles vastly smaller in mass than

Moving rapidly through the space between the nucleus and the edge of the atom, electrons sometimes become dislodged, causing the atom to become a positively charged ion. Conversely, sometimes an atom takes on one or more electrons, thus acquiring a negative charge. Ions are critical to the formation of some kinds of chemical bonds, but the chemical role of the electron is not limited to ionic bonds.
In fact, what defines an atom's ability to bond with another atom, and therefore to form a molecule, is the specific configuration of its electrons. Furthermore, chemical reactions are the result of changes in the arrangement of electrons, not of any activity involving protons or neutrons. So important are electrons to the interactions studied in chemistry that a separate essay has been devoted to them.
What an Atom Is
BASIC ATOMIC STRUCTURE.
The definitions of atoms and elements seems, at first glance, almost circular: an element is a substance made up of only one kind of atom, and an atom is the smallest particle of an element that retains all the chemical and physical properties of the element. In fact, these two definitions do not form a closed loop, as they would if it were stated that an element is something made up of atoms. Every item of matter that exists, except for the subatomic particles discussed in this essay, is made up of atoms. An element, on the other hand, is—as stated in its definition—made up of only one kind of atom. "Kind of atom" in this context refers to the number of protons in its nucleus.
Protons are one of three basic subatomic particles, the other two being electrons and neutrons. As we shall see, there appear to be particles even smaller than these, but before approaching these "sub-subatomic" particles, it is necessary to address the three most significant components of an atom. These are distinguished from one another in terms of electric charge: protons are positively charged, electrons are negative in charge, and neutrons have no electrical charge. As with the north and south poles of magnets, positive and negative charges attract one another, whereas like charges repel. Atoms have no net charge, meaning that the protons and electrons cancel out one another.
EVOLVING MODELS OF THE ATOM.
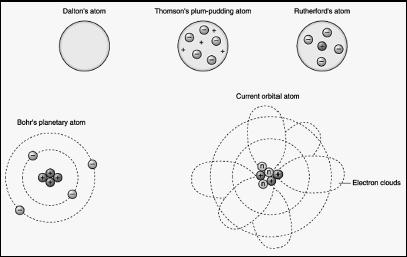
Scientists originally thought of an atom as a sort of closed sphere with a relatively hard shell, rather like a ball bearing. Nor did they initially understand that atoms themselves are divisible, consisting of the parts named above. Even as awareness of these three parts emerged in the last years of the nineteenth century and the first part of the twentieth, it was not at all clear how they fit together.
At one point, scientists believed that electrons floated in a cloud of positive charges. This was before the discovery of the nucleus, where the protons and neutrons reside at the heart of the atom. It then became clear that electrons were moving around the nucleus, but how? For a time, a planetary model seemed appropriate: in other words, electrons revolved around the nucleus much as planets orbit the Sun. Eventually, however—as is often the case with scientific discovery—this model became unworkable, and had to be replaced by another.
The model of electron behavior accepted today depicts the electrons as forming a cloud around the nucleus—almost exactly the opposite of what physicists believed a century ago. The use of the term "cloud" may perhaps be a bit misleading, implying as it does something that simply hovers. In fact, the electron, under normal circumstances, is constantly moving. The paths of its movement around the nucleus are nothing like that of a planet's orbit, except inasmuch as both models describe a relatively small object moving around a relatively large one.
The furthest edges of the electron's movement define the outer perimeters of the atom. Rather than being a hard-shelled little nugget of matter, an atom—to restate the metaphor mentioned above—is a cloud of electrons surrounding a nucleus. Its perimeters are thus not sharply delineated, just as there is no distinct barrier between Earth's atmosphere and space itself. Just as the air gets thinner the higher one goes, so it is with an atom: the further a point is from the nucleus, the less the likelihood that an electron will pass that point on a given orbital path.
Nucleons
MASS NUMBER AND ATOMIC NUMBER.
The term nucleon is used generically to describe the relatively heavy particles that make up an atomic nucleus. Just as "sport" can refer to football, basketball, or baseball, or any other item in a similar class, such as soccer or tennis, "nucleon" refers to protons and neutrons. The sum of protons and neutrons is sometimes called the nucleon number, although a more commonly used term is mass number.
Though the electron is the agent of chemical reactions and bonding, it is the number of protons in the nucleus that defines an atom as to its element. Atoms of the same element always have the same number of protons, and since this figure is unique for a given element, each element is assigned an atomic number equal to the number of protons in its nucleus. The atoms are listed in order of atomic number on the periodic table of elements.
ATOMIC MASS AND ISOTOPES.
A proton has a mass of 1.673 · 10 −24 g, which is very close to the established figure for measuring atomic mass, the atomic mass unit. At one time, the basic unit of atomic mass was equal to the mass of one hydrogen atom, but hydrogen is so reactive—that is, it tends to combine readily with other atoms to form a molecule, and hence a compound—that it is difficult to isolate. Instead, the atomic mass unit is today defined as 1/12 of

The mention of carbon-12, a substance found in all living things, brings up the subject of isotopes. The "12" in carbon-12 refers to its mass number, or the sum of protons and neutrons. Two atoms may be of the same element, and thus have the same number of protons, yet differ in their number of neutrons—which means a difference both in mass number and atomic mass. Such differing atoms of the same element are called isotopes. Isotopes are often designated by symbols showing mass number to the upper left of the chemical symbol or element symbol—for instance, 12 C for carbon-12.
ELECTRIC CHARGE.
Protons have a positive electric charge of 1, designated either as 1+ or +1. Neutrons, on the other hand, have no electric charge. It appears that the 1+ charge of a proton and the 0 charge of a neutron are the products of electric charges on the part of even smaller particles called quarks. A quark may either have a positive electric charge of less than 1+, in which case it is called an "up quark"; or a negative charge of less than 1−, in which case it is called a "down quark."
Research indicates that a proton contains two up quarks, each with a charge of 2/3+, and
A neutron has about the same mass as a proton, but other than its role in forming isotopes, the neutron's function is not exactly clear. Perhaps, it has been speculated, it binds protons—which, due to their positive charges, tend to repel one another—together at the nucleus.
Electrons
An electron is much smaller than a proton or neutron, and has much less mass; in fact, its mass is equal to 1/1836 that of a proton, and 1/1839 that of a neutron. Yet the area occupied by electrons—the region through which they move—constitutes most of the atom's volume. If the nucleus of an atom were the size of a BB (which, in fact, is billions of times larger than a nucleus), the furthest edge of the atom would be equivalent to the highest ring of seats around an indoor sports arena. Imagine the electrons as incredibly fast-moving insects buzzing constantly through the arena, passing by the BB but then flitting to the edges or points in between, and you have something approaching an image of the atom's interior.
How fast does an electron move? Speeds vary depending on a number of factors, but it can move nearly as fast as light: 186,000 mi (299,339 km) per second. On the other hand, for an item of matter near absolute zero in temperature, the velocity of the electron is much, much less. In any case, given the fact that an electron has enough negative charge to cancel out that of the proton, it must be highly energized. After all, this would be like an electric generator weighing 1 lb having as much power as a generator that weighed 1 ton.
According to what modern scientists know or hypothesize concerning the inner structure of the atom, electrons are not made up of quarks; rather, they are part of a class of particles called leptons. It appears that leptons, along with quarks and what are called exchange particles, constitute the elementary particles of atoms—particles on a much more fundamental level than that of the proton and neutron.
Electrons are perhaps the most intriguing parts of an atom. Their mass is tiny, even in atomic terms, yet they possess enough charge to counteract a "huge" proton. They are capable, in certain situations, of moving from one atom to another, thus creating ions, and depending on their highly complex configuration and ability to rearrange their configuration, they facilitate or prevent chemical reactions.
Comment about this article, ask questions, or add new information about this topic: