Gases - How it works

Molecular Motion and Phases of Matter
On Earth, three principal phases or states of matter exist: solid, liquid, and gas. The differences between these three are, on the surface at least, easily perceivable. Clearly water is a liquid, just as ice is a solid and steam a vapor or gas. Yet the ways in which various substances convert between phases are often complex, as are the interrelations between these phases. Ultimately, understanding of the phases depends on an awareness of what takes place at the molecular level.
All molecules are in motion, and the rate of that motion determines the attraction between them. The movement of molecules generates kinetic energy, or the energy of movement, which is manifested as thermal energy. In everyday language, thermal energy is what people mean when they say "heat"; but in scientific terms, heat has a different definition.
The force that attracts atoms to atoms, or molecules to molecules, is not the same as gravitational force, which holds the Moon in orbit around Earth, Earth in orbit around the Sun, and so on. By contrast, the force of interatomic and intermolecular attraction is electromagnetic. Just as the north pole of a magnet is attracted to the south pole of another magnet and repelled by that other magnet's north pole, so positive electric charges are attracted to negative charges, and negatives to positives. (In fact, electricity and magnetism are both manifestations of an electromagnetic interaction.)
The electromagnetic attractions between molecules are much more complex than this explanation makes it seem, and they play a highly significant role in chemical bonding. In simple terms, however, one can say that the greater the rate of motion for the molecules in relation to one another, the less the attraction between molecules. In addition, the kinetic energy, and hence the thermal energy, is greater in a substance whose molecules are relatively free to move.
When the molecules in a material move slowly in relation to one another, they exert a strong attraction, and the material is called a solid. Molecules of liquid, by contrast, move at
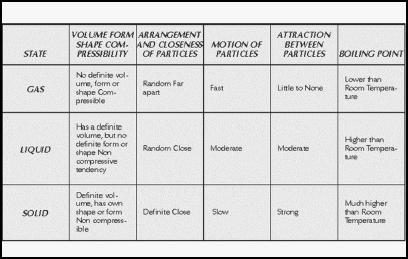
Comparison of Gases to Other Phases of Matter
WATER AND AIR COMPARED.
Gases respond to changes in pressure and temperature in a manner remarkably different from that of solids or liquids. Consider the behavior of liquid water as compared with air—a combination of oxygen (O 2 ), nitrogen (N 2 ), and other gases—in response to experiments involving changes in pressure and temperature.
In the first experiment, both samples are subjected to an increase in pressure from 1 atm (that is, normal atmospheric pressure at sea level) to 2 atm. In the second, both experience an increase in temperature from 32°F (0°C) to 212°F (100°C). The differences in the responses of water and air are striking.
A sample of water that experiences an increase in pressure from 1 to 2 atm will decrease in volume by less than 0.01%, while a temperature increase from the freezing point to the boiling point will result in only a 2% increase in volume. For air, however, an equivalent pressure increase will decrease the volume by a whopping 50%, and an equivalent temperature increase results in a volume increase of 37%.
Air and other gases, by definition, have a boiling point below room temperature. If they did not boil and thus become gas well below ordinary temperatures, they would not be described as substances that are in the gaseous state in most circumstances. The boiling point of water, of course, is higher than room temperature, and that of solids is much higher.
THE ARRANGEMENT OF PARTICLES.
Solids possess a definite volume and a definite shape, and are relatively noncompressible: for instance, if one applies extreme pressure to a steel plate, it will bend, but not much. Liquids have a definite volume, but no definite shape, and tend to be noncompressible. Gases, on the other hand, possess no definite volume or shape, and are highly compressible.
At the molecular level, particles of solids tend to be definite in their arrangement and close in proximity—indeed, part of what makes a solid "solid," in the everyday meaning of that term, is the fact that its constituent parts are basically immovable. Liquid molecules, too, are close in proximity, though random in arrangement. Gas

Pressure
There are a number of statements, collectively known as the "gas laws," that describe and predict the behavior of gases in response to changes in temperature, pressure, and volume. Temperature and volume are discussed elsewhere in this book. However, the subject of pressure requires some attention before we can continue with a discussion of the gas laws.
When a force is applied perpendicular to a surface area, it exerts pressure on that surface. Hence the formula for pressure is p = F / A , where p is pressure, F force, and A the area over which the force is applied. The greater the force, and the smaller the area of application, the greater the pressure; conversely, an increase in area—even without a reduction in force—reduces the overall pressure.
Pressure is measured by a number of units in the English and SI systems. Because p = F / A , all units of pressure represent some ratio of force to surface area.
UNITS OF PRESSURE.
The principal SI unit of pressure is called a pascal (Pa), or 1 N/m 2 . It is named for French mathematician and physicist Blaise Pascal (1623-1662), who is credited with Pascal's principle. The latter holds that the external pressure applied on a fluid—which, in the physical sciences, can mean either a gas or a liquid—is transmitted uniformly throughout the entire body of that fluid.
A newton (N), the SI unit of force, is equal to the force required to accelerate 1 kg of mass at a rate of 1 m/sec 2 . Thus a Pascal is the pressure of 1 newton over a surface area of 1 m 2 . In the English or British system, pressure is measured in terms of pounds per square inch, abbreviated as lbs./in 2 . This is equal to 6.89 · 10 3 Pa, or 6,890 Pa.
Another important measure of pressure is the atmosphere (atm), which is the average pressure exerted by air at sea level. In English units, this is equal to 14.7 lb/in 2 , and in SI units, to 1.013 · 10 5 Pa.
There are two other specialized units of pressure measurement in the SI system: the bar, equal to 10 5 Pa, and the torr, equal to 133 Pa. Meteorologists, scientists who study weather patterns, use the millibar (mb), which, as its name implies, is equal to 0.001 bars. At sea level, atmospheric pressure is approximately 1,013 mb.
The torr, also known as the millimeter of mercury (mm Hg), is the amount of pressure required to raise a column of mercury (chemical symbol Hg) by 1 mm. It is named for Italian physicist Evangelista Torricelli (1608-1647), who invented the barometer, an instrument for measuring atmospheric pressure.
THE BAROMETER.
The barometer constructed by Torricelli in 1643 consisted of a long glass tube filled with mercury. The tube was open at one end, and turned upside down into a dish containing more mercury: the open end was submerged in mercury, while the closed end at the top constituted a vacuum—that is, an area devoid of matter, including air.
The pressure of the surrounding air pushed down on the surface of the mercury in the bowl, while the vacuum at the top of the tube provided an area of virtually no pressure into which the mercury could rise. Thus the height to which the

The value of 1 atm was thus established asequal to the pressure exerted on a column ofmercury 760 mm high at a temperature of 0°C(32°F). In time, Torricelli's invention became afixture both of scientific laboratories and ofhouseholds. Since changes in atmospheric pressure have an effect on weather patterns, manyhome indoor-outdoor thermometers today alsoinclude a barometer.
Comment about this article, ask questions, or add new information about this topic: