Properties of Matter - Real-life applications

Types of Solids
Particles of solids resist attempts to compress them, or push them together, and because of their close proximity, solid particles are fixed in an orderly and definite pattern. As a result, a solid usually has a definite volume and shape.
A crystalline solid is a type of solid in which the constituent parts are arranged in a simple, definite geometric pattern that is repeated in all directions. But not all crystalline solids are the same. Table salt is an example of an ionic solid: a form of crystalline solid that contains ions. When mixed with a solvent such as water, ions from the salt move freely throughout the solution, making it possible to conduct an electric current.
Regular table sugar (sucrose) is a molecular solid, or one in which the molecules have a neutral electric charge—that is, there are no ions present. Therefore, a solution of water and sugar would not conduct electricity. Finally, there are crystalline solids known as atomic solids, in which atoms of one element bond to one another. Examples include diamonds (made of pure carbon), silicon, and all metals.
Other solids are said to be amorphous, meaning that they possess no definite shape. Amorphous solids—an example of which is clay—either possess very tiny crystals, or consist of several varieties of crystal mixed randomly. Still other solids, among them glass, do not contain crystals.
Freezing and Melting
VIBRATIONS AND FREEZING.
Because of their slow movement in relation to one another, solid particles exert strong attractions; yet as slowly as they move, solid particles do move—as is the case with all forms of matter at the atomic level. Whereas the particles in a liquid or gas move fast enough to be in relative motion with regard to one another, however, solid particles merely vibrate from a fixed position.
As noted earlier, the motion and attraction of particles in matter has a direct effect on thermal energy, and thus on heat and temperature. The cooler the solid, the slower and weaker the vibrations, and the closer the particles are to one another. Thus, most types of matter contract when freezing, and their density increases. Absolute zero, or 0K on the Kelvin scale of temperature—equal to −459.67°F (−273°C)—is the point at which vibration virtually ceases.
Note that the vibration virtually stops, but does not totally stop. In fact, as established in the third law of thermodynamics, absolute zero is impossible to achieve: thus, the relative motion of molecules never ceases. The lowest temperature actually achieved, at a Finnish nuclear laboratory in 1993, is 2.8 · 10 −10 K, or 0.00000000028K—still above absolute zero.
UNUSUAL CHARACTERISTICS OF SOLID AND LIQUID WATER.
The behavior of water when frozen is interesting and exceptional. Above 39.2°F (4°C) water, like most substances, expands when heated. In other words, the molecules begin moving further apart as expected, because—in this temperature range, at least—water behaves like other substances, becoming "less solid" as the temperature increases.
Between 32°F (0°C) and 39.2°F (4°C), however, water actually contracts. In this temperature range, it is very "cold" (that is, it has relatively little heat), but it is not frozen. The density of water reaches its maximum—in other words, water molecules are as closely packed as they can be—at 39.2°F; below that point, the density starts to decrease again. This is highly unusual: in most substances, the density continues to increase with lowered temperatures, whereas water is actually most dense slightly above the freezing point.
Below the freezing point, then, water expands, and therefore when water in pipes freezes, it may increase in volume to the point where it bursts the pipe. This is also the reason why ice floats on water: its weight is less than that of the water it has displaced, and thus it is buoyant. Additionally, the buoyant qualities of ice atop very cold water helps explain the behavior of lake water in winter; although the top of a lake may freeze, the entire lake rarely freezes solid—even in the coldest of inhabited regions.
Instead of freezing from the bottom up, as it would if ice were less buoyant than the water, the lake freezes from the top down—an important thing to remember when ice-fishing! Furthermore, water in general (and ice in particular) is a poor conductor of heat, and thus little of the heat from the water below it escapes. Therefore, the lake does not freeze completely—only a layer at the top—and this helps preserve animal and plant life in the body of water.
MELTING.
When heated, particles begin to vibrate more and more, and therefore move further apart. If a solid is heated enough, it loses its rigid structure and becomes a liquid. The temperature at which a solid turns into a liquid is called the melting point, and melting points are different for different substances. The melting point of a substance, incidentally, is the same as its freezing point: the difference is a matter of orientation—that is, whether the process is one of a solid melting to become a liquid, or of a liquid freezing to become a solid.
The energy required to melt 1 mole of a solid substance is called the molar heat of fusion. It can be calculated by the formula Q = smδT, where Q is energy, s is specific heat capacity, m is mass, and δT means change in temperature. (In the symbolic language often employed by scientists, the Greek letter δ, or delta, stands for "change in.") Specific heat capacity is measured in units of J/g · °C (joules per gram-degree Celsius), and energy in joules or kilojoules (kJ)—that is, 1,000 joules.
In melting, all the thermal energy in a solid is used in breaking up the arrangement of crystals, called a lattice. This is why water melted from ice does not feel any warmer than the ice did: the thermal energy has been expended, and there is none left over for heating the water. Once all the ice is melted, however, the absorbed energy from the particles—now moving at much greater speeds than when the ice was in a solid state—causes the temperature to rise.
For the most part, solids composed of particles with a higher average atomic mass require more energy—and hence higher temperatures—to induce the vibrations necessary for melting. Helium, with an average atomic mass of 4.003 amu, melts or freezes at an incredibly low temperature: −457.6°F (−272°C), or close to absolute zero. Water, for which, as noted earlier, the average atomic mass is the sum of the masses for its two hydrogen atoms and one oxygen atom, has an average molecular mass of 18.016 amu. Ice melts (or water freezes) at much higher temperatures than helium: 32°F (0°C). Copper, with an average atomic mass of 63.55 amu, melts at much, much higher temperatures than water: 1,985°F (1,085°C).
Liquids
The particles of a liquid, as compared to those of a solid, have more energy, more motion, and—generally speaking—less attraction to one another. The attraction, however, is still fairly strong: thus, liquid particles are in close enough proximity that the liquid resists attempts at compression.
On the other hand, their arrangement is loose enough that the particles tend to move around one another rather than simply vibrate in place the way solid particles do. A liquid is therefore not definite in shape. Due to the fact that the particles in a liquid are farther apart than those of a solid, liquids tend to be less dense than solids. The liquid phase of a substance thus tends to be larger in volume than its equivalent in solid form. Again, however, water is exceptional in this regard: liquid water actually takes up less space than an equal mass of frozen water.
Boiling
When a liquid experiences an increase in temperature, its particles take on energy and begin to move faster and faster. They collide with one another, and at some point the particles nearest the surface of the liquid acquire enough energy to break away from their neighbors. It is at this point that the liquid becomes a gas or vapor.
As heating continues, particles throughout the liquid begin to gain energy and move faster, but they do not immediately transform into gas. The reason is that the pressure of the liquid, combined with the pressure of the atmosphere above the liquid, tends to keep particles in place. Those particles below the surface, therefore, remain where they are until they acquire enough energy to rise to the surface.
The heated particle moves upward, leaving behind it a hollow space—a bubble. A bubble is not an empty space: it contains smaller trapped particles, but its small mass, relative to that of the liquid it disperses, makes it buoyant. Therefore, a bubble floats to the top, releasing its trapped particles as gas or vapor. At that point, the liquid is said to be boiling.
THE EFFECT OF ATMOSPHERIC PRESSURE.
The particles thus have to overcome atmospheric pressure as they rise, which means that the boiling point for any liquid depends in part on the pressure of the surrounding air. Normal atmospheric pressure (1 atm) is equal to 14 lb/in 2 (1.013 × 10 5 Pa), and is measured at sea level. The greater the altitude, the less the air pressure, because molecules of air—since air is a gas, and therefore its particles are fast-moving and non-attractive—respond less to Earth's gravitational pull. This is why airplanes require pressurized cabins to maintain an adequate oxygen supply; but even at altitudes much lower than the flight path of an airplane, differences in air pressure are noticeable.
It is for this reason that cooking instructions often vary with altitude. Atop Mt. Everest, Earth's highest peak at about 29,000 ft (8,839 m) above sea level, the pressure is approximately one-third of normal atmospheric pressure. Water boils at a much lower temperature on Everest than it does elsewhere: 158°F (70°C), as opposed to 212°F (100°C) at sea level. Of course, no one lives on the top of Mt. Everest—but people do live in Denver, Colorado, where the altitude is 5,577 ft (1,700 m) and the boiling point of water is 203°F (95°C).
Given the lower boiling point, one might assume that food would cook faster in Denver than in New York, Los Angeles, or in any city close to sea level. In fact, the opposite is true: because heated particles escape the water so much faster at high altitudes, they do not have time to acquire the energy needed to raise the temperature of the water. It is for this reason that a recipe may include a statement such as "at altitudes above XX feet, add XX minutes to cooking time."
If lowered atmospheric pressure means a lowered boiling point, what happens in outer space, where there is no atmospheric pressure? Liquids boil at very, very low temperatures. This is one of the reasons why astronauts have to wear pressurized suits: if they did not, their blood would boil—even though space itself is incredibly cold.
LIQUID TO GAS AND BACK AGAIN.
Note that the process of changing a liquid to a gas is similar to that which occurs when a solid changes to a liquid: particles gain heat and therefore energy, begin to move faster, break free from one another, and pass a certain threshold into a new phase of matter. And just as the freezing and melting point for a given substance are the same temperature—the only difference being one of orientation—the boiling point of a liquid transforming into a gas is the same as the condensation point for a gas turning into a liquid.
The behavior of water in boiling and condensation makes possible distillation, one of the principal methods for purifying sea water in various parts of the world. First the water is boiled, then it is allowed to cool and condense, thus forming water again. In the process, the water separates from the salt, leaving it behind in the form of brine. A similar separation takes place when salt water freezes: because salt, like most crystalline solids, has a much lower freezing point than water, very little of it remains joined to the water in ice. Instead, the salt takes the form of a briny slush.
Gases
A liquid that is vaporized, or any substance that exists normally as a gas, is quite different in physical terms from a solid or a liquid. This is illustrated by the much higher energy component in the molar heat of vaporization, or the amount of energy required to turn 1 mole of a liquid into a gas.
Consider, for instance, what happens to water when it experiences phase changes. Assuming that heat is added at a uniform rate, when ice reaches its melting point, there is only a relatively small period of time when the H 2 O is composed of both ice and liquid. But when the liquid reaches its boiling point, the water is present both as a liquid and a vapor for a much longer period of time. In fact, it takes almost seven times as much energy to turn liquid water into pure steam than it does to turn ice into purely liquid water. Thus, the molar heat of fusion for water is 6.02 kJ/mol, while the molar heat of vaporization is 40.6 kJ/mol.
Although liquid particles exert a moderate attraction toward one another, particles in a gas (particularly a substance that normally exists as a gas at ordinary temperatures on Earth) exert little to no attraction. They are thus free to move, and to move quickly. The overall shape and arrangement of gas is therefore random and indefinite—and, more importantly, the motion of gas particles provides much greater kinetic energy than is present in any other major form of matter on Earth.
The constant, fast, and random motion of gas particles means that they are regularly colliding and thereby transferring kinetic energy back and forth without any net loss of energy. These collisions also have the overall effect of producing uniform pressure in a gas. At the same time, the characteristics and behavior of gas particles indicate that they tend not to remain in an open container. Therefore, in order to have any pressure on a gas—other than normal atmospheric pressure—it is necessary to keep it in a closed container.
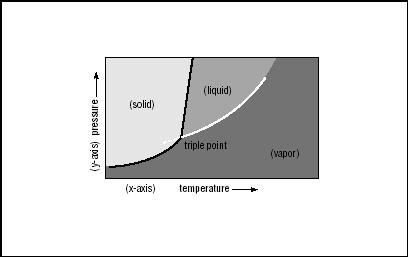
The Phase Diagram
The vaporization of water is an example of a change of phase—the transition from one phase of matter to another. The properties of any substance, and the points at which it changes phase, are plotted on what is known as a phase diagram. The phase diagram typically shows temperature along the x-axis, and pressure along the y-axis.
For simple substances, such as water and carbon dioxide (CO 2 ), the solid form of the substance appears at a relatively low temperature and at pressures anywhere from zero upward. The line between solids and liquids, indicating the temperature at which a solid becomes a liquid at any pressure above a certain level, is called the fusion curve. Though it appears to be a more or less vertical line, it is indeed curved, indicating that at high pressures, a solid well below the normal freezing point may be melted to create a liquid.
Liquids occupy the area of the phase diagram corresponding to relatively high temperatures and high pressures. Gases or vapors, on the other hand, can exist at very low temperatures, but only if the pressure is also low. Above the melting point for the substance, gases exist at higher pressures and higher temperatures. Thus, the line between liquids and gases often looks almost like a 45° angle. But it is not a straight line, as its name, the vaporization curve, implies. The curve of vaporization demonstrates that at relatively high temperatures and high pressures, a substance is more likely to be a gas than a liquid.
THE CRITICAL POINT.
There are several other interesting phenomena mapped on a phase diagram. One is the critical point, found at a place of very high temperature and pressure along the vaporization curve. At the critical point, high temperatures prevent a liquid from remaining a liquid, no matter how high the pressure.
At the same time, the pressure causes gas beyond that point to become increasingly more dense, but due to the high temperatures, it does not condense into a liquid. Beyond the critical point, the substance cannot exist in anything other than the gaseous state. The temperature component of the critical point for water is 705.2°F (374°C)—at 218 atm, or 218 times ordinary atmospheric pressure. For helium, however, critical temperature is just a few degrees above absolute zero. This is, in part, why helium is rarely seen in forms other than a gas.
THE SUBLIMATION CURVE.
Another interesting phenomenon is the sublimation curve, or the line between solid and gas. At certain very low temperatures and pressures, a substance may experience sublimation, meaning that a gas turns into a solid, or a solid into a gas, without passing through a liquid stage.
A well-known example of sublimation occurs when "dry ice," made of carbon dioxide, vaporizes at temperatures above (−78.5°C). Carbon dioxide is exceptional, however, in that it experiences sublimation at relatively high pressures that occur in everyday life: for most substances, the sublimation point transpires at such a low pressure point that it is seldom witnessed outside of a laboratory.
THE TRIPLE POINT.
The phenomenon known as the triple point shows how an ordinary substance such as water or carbon dioxide can actually be a liquid, solid, and vapor—all at once. Most people associate water as a gas or vapor (that is, steam) with very high temperatures. Yet, at a level far below normal atmospheric pressure, water can be a vapor at temperatures as low as −4°F (−20 °C). (All of the pressure values in the discussion of water at or near the triple point are far below atmospheric norms: the pressure at which water turns into a vapor at −4°F, for instance, is about 0.001 atm.)
Just as water can exist as a vapor at low temperatures and low pressures, it is also possible for water at temperatures below freezing to remain liquid. Under enough pressure, ice melts and is thereby transformed from a solid to a liquid, at temperatures below its normal freezing point. On the other hand, if the pressure of ice falls below a very low threshold, it will sublimate.
The phase diagram of water shows a line between the solid and liquid states that is almost, but not quite, exactly perpendicular to the x-axis. But in fact, it is a true fusion curve: it slopes slightly upward to the left, indicating that solid ice turns into water with an increase of pressure. Below a certain level of pressure is the vaporization curve, and where the fusion curve intersects the vaporization curve, there is a place called the triple point. Just below freezing, in conditions equivalent to about 0.007 atm, water is a solid, liquid, and vapor all at once.
Other States of Matter
PLASMA.
Principal among states of matter other than solid, liquid, and gas is plasma, which is similar to gas. (The term "plasma," when referring to the state of matter, has nothing to do with the word as it is often used, in reference to blood plasma.) As with gas, plasma particles collide at high speeds—but in plasma the speeds are even greater, and the kinetic energy levels even higher.
The speed and energy of these collisions is directly related to the underlying property that distinguishes plasma from gas. So violent are the collisions between plasma particles that electrons are knocked away from their atoms. As a result, plasma does not have the atomic structure typical of a gas; rather, it is composed of positive ions and electrons. Plasma particles are thus electrically charged, and therefore greatly influenced by electric and magnetic fields.
Formed at very high temperatures, plasma is found in stars. The reaction between plasma and atomic particles in the upper atmosphere is responsible for the aurora borealis, or "northern lights." Though found on Earth only in very small quantities, plasma—ubiquitous in other parts of the universe—may be the most plentiful of all the states of matter.
QUASI-STATES.
Among the quasi-states of matter discussed by scientists are several terms describing the structure in which particles are joined, rather than the attraction and relative movement of those particles. Thus "crystalline," "amorphous," and "glassy" are all terms to describe what may be individual states of matter; so too is "colloidal."
A colloid is a structure intermediate in size between a molecule and a visible particle, and it has a tendency to be dispersed in another medium—the way smoke, for instance, is dispersed in air. Brownian motion describes the behavior of most colloidal particles. When one sees dust floating in a ray of sunshine through a window, the light reflects off colloids in the dust, which are driven back and forth by motion in the air otherwise imperceptible to the human senses.
DARK MATTER.
The number of states or phases of matter is clearly not fixed, and it is quite possible that more will be discovered in outer space, if not on Earth. One intriguing candidate is called dark matter, so described because it neither reflects nor emits light, and is therefore invisible. In fact, luminous or visible matter may very well make up only a small fraction of the mass in the universe, with the rest being taken up by dark matter.
If dark matter is invisible, how do astronomers and physicists know it exists? By analyzing the gravitational force exerted on visible objects in such cases where there appears to be no visible object to account for that force. An example is the center of our galaxy, the Milky Way. It appears to be nothing more than a dark "halo," but in order to cause the entire galaxy to revolve around it—in the same way that planets revolve around the Sun, though on a vastly larger scale—it must contain a staggering quantity of invisible mass.
The Bose-Einstein Condensate
Physicists at the Joint Institute of Laboratory Astrophysics in Boulder, Colorado, in 1995 revealed a highly interesting aspect of atomic behavior at temperatures approaching absolute zero. Some 70 years before, Einstein had predicted that, at extremely low temperatures, atoms would fuse to form one large "superatom." This hypothesized structure was dubbed the Bose-Einstein Condensate (BEC) after Einstein and Satyendranath Bose (1894-1974), an Indian physicist whose statistical methods contributed to the development of quantum theory.
Cooling about 2,000 atoms of the element rubidium to a temperature just 170 billionths of a degree Celsius above absolute zero, the physicists succeeded in creating an atom 100 micrometers across—still incredibly small, but vast in comparison to an ordinary atom. The superatom, which lasted for about 15 seconds, cooled down all the way to just 20 billionths of a degree above absolute zero. The Colorado physicists won the Nobel Prize in physics in 1997 for their work.
In 1999, researchers in a lab at Harvard University also created a superatom of BEC, and used it to slow light to just 38 MPH (61.2 km/h)—about 0.02% of its ordinary speed. Dubbed a "new" form of matter, the BEC may lead to a greater understanding of quantum mechanics, and may aid in the design of smaller, more powerful computer chips.
Some Unusual Phase Transitions
At places throughout this essay, references have been made variously to "phases" and "states" of matter. This is not intended to confuse, but rather to emphasize a particular point. Solids, liquids, and gases are referred to as "phases" because many (though far from all) substances on Earth regularly move from one phase to another.
There is absolutely nothing incorrect in referring to "states of matter." But "phases of matter" is used in the present context as a means of emphasizing the fact that substances, at the appropriate temperature and pressure, can be solid, liquid, or gas. The phases of matter, in fact, can be likened to the phases of a person's life: infancy, babyhood, childhood, adolescence, adulthood, old age. The transition between these stages is indefinite, yet it is easy enough to say when a person is at a certain stage.
LIQUID CRYSTALS.
A liquid crystal is a substance that, over a specific range of temperature, displays properties both of a liquid and a solid. Below this temperature range, it is unquestionably a solid, and above this range it is just as certainly a liquid. In between, however, liquid crystals exhibit a strange solid-liquid behavior: like a liquid, their particles flow, but like a solid, their molecules maintain specific crystalline arrangements.
The cholesteric class of liquid crystals is so named because the spiral patterns of light through the crystal are similar to those which appear in cholesterols. Depending on the physical properties of a cholesteric liquid crystal, only certain colors may be reflected. The response of liquid crystals to light makes them useful in liquid crystal displays (LCDs) found on laptop computer screens, camcorder views, and in other applications.
LIQUEFACTION OF GASES.
One interesting and useful application of phase change is the liquefaction of gases, or the change of gas into liquid by the reduction in its molecular energy levels. Liquefied natural gas (LNG) and liquefied petroleum gas (LPG), the latter a mixture of by-products obtained from petroleum and natural gas, are among the examples of liquefied gas in daily use. In both cases, the volume of the liquefied gas is far less than it would be if the gas were in a vaporized state, thus enabling ease and economy of transport.
Liquefied gases are used as heating fuel for motor homes, boats, and homes or cabins in remote areas. Other applications of liquefied gases include liquefied oxygen and hydrogen in rocket engines; liquefied oxygen and petroleum used in welding; and a combination of liquefied oxygen and nitrogen used in aqualung devices. The properties of liquefied gases figure heavily in the science of producing and studying low-temperature environments. In addition, liquefied helium is used in studying the behavior of matter at temperatures close to absolute zero.
COAL GASIFICATION.
Coal gasification, as one might discern from the name, is the conversion of coal to gas. Developed before World War II, it fell out of favor after the war, due to the lower cost of oil and natural gas. However, increasingly stringent environmental regulations imposed by the federal government on industry during the 1970s, combined with a growing concern for the environment on the part of the populace as a whole, led to a resurgence of interest in coal gasification.
Though widely used as a fuel in power plants, coal, when burned by ordinary means, generates enormous air pollution. Coal gasification, on the other hand, makes it possible to burn "clean" coal. Gasification involves a number of chemical reactions, some exothermic or heat-releasing, and some endothermic or heat-absorbing. At one point, carbon monoxide is released in an exothermic reaction, then mixed with hydrogen released from the coal to create a second exothermic reaction. The energy discharged in these first two reactions is used to initiate a third, endothermic, reaction.
The finished product of coal gasification is a mixture containing carbon monoxide, methane, hydrogen, and other substances, and this—rather than ordinary coal—is burned as a fuel. The composition of the gases varies according to the process used. Products range from coal synthesis gas and medium-Btu gas (both composed of carbon monoxide and hydrogen, though combined in different forms) to substitute natural gas, which consists primarily of methane.
Not only does coal gasification produce a clean-burning product, but it does so without the high costs associated with flue-gas desulfurization systems. The latter, often called "scrubbers," were originally recommended by the federal government to industry, but companies discovered that coal gasification could produce the same results for much less money. In addition, the waste products from coal gasification can be used for other purposes. At the Cool Water Integrated Gasification Combined Cycle Plant, established in Barstow, California, in 1984, sulfur obtained from the reduction of sulfur dioxide is sold off for about $100 a ton.
The Chemical Dimension to Changes of Phase
Throughout much of this essay, we have discussed changes of phase primarily in physical terms; yet clearly these changes play a significant role in chemistry. Furthermore, coal gasification serves to illustrate the impact chemical processes can have on changes of state.
Much earlier, figures were given for the melting points of copper, water, and helium, and these were compared with the average atomic mass of each. Those figures, again, are:
Average Atomic Mass and Melting Points of a Sample Gas, Liquid, and Solid
- Helium: 4.003 amu; −457.6°F (−210°C)
- Water: 40.304 amu 32°F (0°C)
- Copper: 63.55 amu; 1,985°F (1,085°C).
Something seems a bit strange about those comparisons: specifically, the differences in melting point appear to be much more dramatic than the differences in average atomic mass. Clearly, another factor is at work—a factor that relates to the difference in the attractions between molecules in each. Although the differences between solids, liquids, and gases are generally physical, the one described here—a difference between substances—is clearly chemical in nature.
To discuss this in the detail it deserves would require a lengthy digression on the chemical dimensions of intermolecular attraction. Nonetheless, it is possible here to offer at least a cursory answer to the question raised by these striking differences in response to temperature.
Dipoles, Electron Seas, and London Dispersion
Water molecules are polar, meaning that one area of a water molecule is positively charged, while another area has a negative charge. Thus the positive side of one molecule is drawn to the negative side of another, and vice versa, which gives water a much stronger intermolecular bond than, for instance, oil, in which the positive and negative charges are evenly distributed throughout the molecule.
Yet the intermolecular attraction between the dipoles (as they are called) in water is not nearly as strong as the bond that holds together a metal. Particles in copper or other metals "float" in a tightly packed "sea" of highly mobile electrons, which provide a bond that is powerful, yet lacking in a firm directional orientation. Thus metals are both strong and highly malleable (that is, they can be hammered very flat without breaking.)
Water, of course, appears most often as a liquid, and copper as a solid, precisely because water has a very high boiling point (the point at which it becomes a vapor) and copper has a very high melting point. But consider helium, which has the lowest freezing point of any element: just above absolute zero. Even then, a pressure equal to 25 times that of normal atmospheric pressure is required to push it past the freezing point.
Helium and other Group 8 or Group 18 elements, as well as non-polar molecules such as oils, are bonded by what is called London dispersion forces. The latter, as its name suggests, tends to keep molecules dispersed, and induces instantaneous dipoles when most of the electrons happen to be on one side of an atom. Of course, this happens only for an infinitesimal fraction of time, but it serves to create a weak attraction. Only at very low temperatures do London dispersion forces become strong enough to result in the formation of a solid.
WHERE TO LEARN MORE
Biel, Timothy L. Atom: Building Blocks of Matter. San Diego, CA: Lucent Books, 1990.
Feynman, Richard. Six Easy Pieces: Essentials of Physics Explained by Its Most Brilliant Teacher. New introduction by Paul Davies. Cambridge, MA: Perseus Books, 1995.
"High School Chemistry Table of Contents—Solids and Liquids" Homeworkhelp.com (Web site). <http://www.homeworkhelp.com/homeworkhelp/freemember/text/chem/high/topic09.htm> (April 10, 2001).
"Matter: Solids, Liquids, Gases." Studyweb (Web site). <http://www.studyweb.com/links/4880.html> (April 10, 2001).
"The Molecular Circus" (Web site). <http://www.cpo.com/Weblabs/circus.htm> (April 10, 2001).
Paul, Richard. A Handbook to the Universe: Explorations of Matter, Energy, Space, and Time for Beginning Scientific Thinkers. Chicago: Chicago Review Press, 1993.
"Phases of Matter" (Web site). <http://pc65.frontier.osrhe.edu/hs/science/pphase.htm> (April 10, 2001).
Royston, Angela. Solids, Liquids, and Gasses. Chicago: Heinemann Library, 2001.
Wheeler, Jill C. The Stuff Life's Made Of: A Book About Matter. Minneapolis, MN: Abdo & Daughters Publishing, 1996.
Zumdahl, Steven S. Introductory Chemistry: A Foundation, 4th ed. Boston: Houghton Mifflin, 2000.