Chemical Bonding - How it works
Early Ideas of Bonding
The theory that all of matter is composed of atoms did not originate in modern times: the atomic model actually dates back to the fifth century B.C. in Greece. The leading exponent of atomic theory in ancient times was Democritus (c. 460-370 B.C. ), who proposed that matter could not be infinitely subdivided. At its deepest substructure, Democritus maintained, the material world was made up of tiny fragments he called atomos , a Greek term meaning "no cut" or "indivisible"
Forward-thinking though it was, Democritus's idea was not what modern scientists today would describe as a proper scientific hypothesis. His "atoms" were not purely physical units, but rather idealized philosophical constructs, and thus, he was not really approaching the subject from the perspective of a scientist. In any case, there was no way for Democritus to test his hypothesis even if he had wanted to: by their very nature, the atoms he described were far too small to observe. Even today, what scientists know about atomic behavior comes not from direct observation, but indirect means.
Hence, Democritus and the few other ancients who subscribed to atomic theory went more on instinct than by scientific methods. Yet, some of them were remarkably prescient in their description of the bonding of atoms, in view of the primitive scientific methods they had at their disposal. No other scientist came close to the accuracy of their theory for about 2,000 years.
ASCLEPIADES AND LUCRETIUS DISCUSS BONDS.
The physician Asclepiades of Prusa (c.130-40 B.C. ) drew on the ideas of the Greek philosopher Epicurus (341-270 B.C. , another proponent of atomism. Asclepiades speculated on the ways in which atoms interact, and discussed "clusters of atoms," though, of course, he had no idea what force attracted the atoms to one another.
A few years after Asclepiades, the Roman philosopher and poet Lucretius (c.95-c.55 B.C. espoused views that combined atomism with the idea of the "four elements"—earth, air, fire, and water. In his great work De rerum natura ("On the Nature of Things"), Lucretius described atoms as tiny spheres attached to each other by fishhook-like appendages that became entangled with one another.
LACK OF PROGRESS UNTIL 1800.
Unfortunately, the competing idea of the four elements, handed down by the great philosopher Aristotle (384-322 B.C. ), prevailed over the atomic model. As the Roman Empire
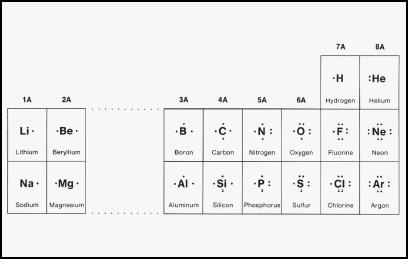
During the seventeenth century, a mounting array of facts from the realms of astronomy and physics collectively disproved the Aristotelian model. In the area of chemistry, English physicist and chemist Robert Boyle (1627-1691) showed that the four elements were not elements at all, because they could be broken down into simpler substances. Yet, no one really understood what constituted an element until the very beginning of the nineteenth century, and until that question was addressed, it was difficult to move on to the mystery of why certain atoms bonded with one another.
Early Modern Advances in Bonding Theory
DALTON'S ATOMIC THEORY.
The birth of atomic theory in modern times occurred in 1803, when English chemist John Dalton (1766-1844) formulated the idea that all elements are composed of tiny, indestructible particles. These he called by the name Democritus had given them nearly 23 centuries earlier: atoms. All known substances, he said, are composed of some combination of atoms, which differ from one another only in mass.
Though Dalton's theory paved the way for enormous advances in the years that followed, there were a number of flaws in it. Mass alone, for instance, is not really what differentiates one atom from another: differences in mass reflect the presence of subatomic particles—protons and neutrons—of whose existence scientists were unaware at the time.
Furthermore, the properties of atoms that cause them to bond relate to a third subatomic particle, the electron, which, though it contributes little to the mass of the atom, is all-important to the energy it possesses. As for how atoms bond to one another, Dalton had little to say: in his conception of the atomic model, atoms simply sit adjacent to one another without forming true bonds, as such.
AVOGADRO AND THE MOLECULE.
Though Dalton recognized that the structure of atoms in a particular element or compound is uniform, he maintained that compounds are made up of compound atoms: thus,
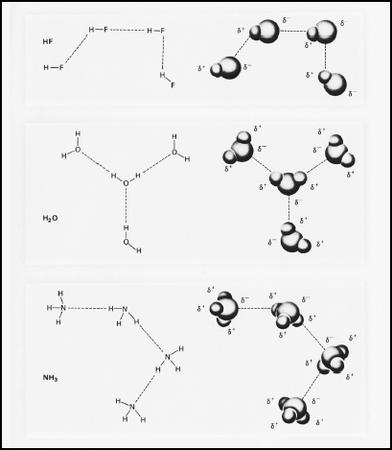
That structure was the molecule, first described by Italian physicist Amedeo Avogadro (1776-1856). For several decades, Avogadro, who originated the idea of the mole as a means of comparing large groups of atoms or molecules, remained a more or less unsung hero. Only in 1860, four years after his death, was his idea of the molecule resurrected by Italian chemist Stanislao Cannizzaro (1826-1910). Cannizzaro's work was occasioned by disagreement among scientists regarding the determination of atomic mass; however, the establishment of the molecular model had far-reaching implications for theories of bonding.
SYMBOLIZING ATOMIC BONDS.
In 1858, German chemist Friedrich August Kekulé (1829-1896) made the first attempt to define the concept of valency, or the property an atom of one element possesses that determines

This was one of the first attempts to examine the subject of bonding using modern scientific terminology, complete with hypotheses that could be tested by experimentation. Kekulé also recognized that in order to discuss bonds understandably, there needed to be some means of representing those bonds with symbols. He even went so far as to develop a system for showing the arrangement of bonds in space; however, his system was so elaborate that it was replaced in favor of a simpler one developed by Scottish chemist Archibald Scott Couper (1831-1892).
Couper, who also studied valency and the tetravalent carbon bond—he is usually given equal credit with Kekulé for these ideas—created an extremely straightforward schematic representation still in use by chemists today. In Couper's system, short dashed lines serve to designate chemical bonds. Hence, the bond between two hydrogen atoms and an oxygen atom in a water molecule would be represented thus: H-O-H. As the understanding of bonds progressed in modern times, this system was modified to take into account multiple bonds, discussed below.
Comment about this article, ask questions, or add new information about this topic: