Osmosis - How it works
Water and Oil: Molecular Differences
In the illustrations involving the beaker and the hollow tube, water plays one of its leading roles, as a solvent. It is possible to use a number of other solvents for osmosis, but most of the ones that will be discussed here are water-based substances. In fact, virtually everything people drink is either made with water as its central component (soft drinks, coffee, tea, beer and spirits), or comes from a water-based plant or animal life form (fruit juices, wine, milk.) Then of course there is water itself, still the world's most popular drink.
By contrast, people are likely to drink an oily product only in extreme circumstances: for instance, to relieve constipation, holistic-health practitioners often recommend a mixture of olive oil and other compounds for this purpose. Oil, unlike water, has a tendency to pass straight through a person's system, without large amounts of it being absorbed through osmosis. In fact, oil and water differ significantly at the molecular level.
Water is the best example of a polar molecule, sometimes called a dipole. As everyone knows, water is a name for the chemical H 2 O, in which two relatively small hydrogen atoms bond with a large atom of oxygen. You can visualize a water molecule by imagining oxygen as a basketball with hydrogen as two baseballs fused to the
As a result, one end of a water molecule has a positive electrical charge, and the other end a negative charge. This in turn causes the positive end of one molecule to attract the negative side of its neighbor, and vice versa. Though the electromagnetic force is weak, even in relative terms, it is enough to bond water molecules tightly to one another.
By contrast, oily substances—whether the oil is animal-, vegetable-, or petroleum-based—are typically nonpolar, meaning that the positive and negative charges are distributed evenly across the surface of the molecule. Hence, the bond between oil molecules is much less tight than for water molecules. Clean motor oil in a car's crankshaft behaves as though it were made of millions of tiny ball-bearings, each rolling through the engine without sticking. Water, on the other hand, has a tendency to stick to surfaces, since its molecules are so tightly bonded to one another.
This tight bond gives water highly unusual properties compared to other substances close to its molecular weight. Among these are its high boiling point, its surprisingly low density when frozen, and the characteristics that make osmosis possible. Thanks to its intermolecular structure, water is not only an ideal solvent, but its closely
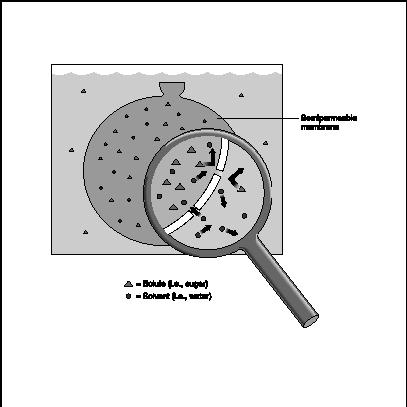
In the beaker illustration, the "pure" water is almost pure solvent. (Actually, because of its solvent qualities, water seldom appears in a pure state unless one distills it: even water flowing through a "pure" mountain stream carries all sorts of impurities, including microscopic particles of the rocks over which it flows.) In any case, the fact that the water in the beaker is almost pure makes it easy for it to flow through the semipermeable membrane in the bottom of the tube. By contrast, the solute particles in the salt-water solution have a much harder time passing through, and are much more likely to block the openings in the membrane. As a result, the movement is all in one direction: water in the beaker moves through the membrane, and into the tube.
A few points of clarification are in order here. A semipermeable membrane is anything with a structure somewhere between that of, say, plastic on the one hand and cotton on the other. Were the tube in the beaker covered with Saran wrap, for instance, no water would pass through. On the other hand, if one used a piece of cotton in the bottom of the tube, the water would pass straight through without osmosis taking place. In contrast to cotton, Gore-tex is a fabric containing a very thin layer of plastic with billions of tiny pores which let water vapor flow out without allowing liquid water to seep in. This accounts for the popularity of Gore-tex for outdoor gear—it keeps a person dry without holding in their sweat. So Gore-tex would work well as a semipermeable membrane.
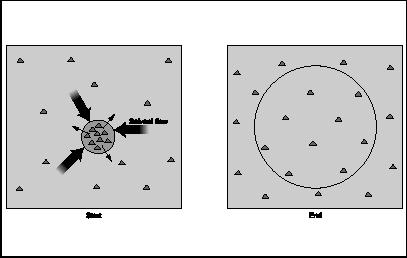
Also, it is important to consider the possibilities of what can happen during the process of osmosis. If the tube were filled with pure salt, or salt with only a little water in it, osmosis would reach a point and then stop due to osmotic pressure within the substance. Osmotic pressure results when a relatively concentrated substance takes in a solvent, thus increasing its pressure until it reaches a point at which the solution will not allow any more solvent to enter.
Comment about this article, ask questions, or add new information about this topic: