AMINO ACIDS
CONCEPT
Amino acids are organic compounds made of carbon, hydrogen, oxygen, nitrogen, and (in some cases) sulfur bonded in characteristic formations. Strings of amino acids make up proteins, of which there are countless varieties. Of the 20 amino acids required for manufacturing the proteins the human body needs, the body itself produces only 12, meaning that we have to meet our requirements for the other eight through nutrition. This is just one example of the importance of amino acids in the functioning of life. Another cautionary illustration of amino acids' power is the gamut of diseases (most notably, sickle cell anemia) that impair or claim the lives of those whose amino acids are out of sequence or malfunctioning. Once used in dating objects from the distant past, amino acids have existed on Earth for at least three billion years—long before the appearance of the first true organisms.
HOW IT WORKS
A "MAP" OF AMINO ACIDS
Amino acids are organic compounds, meaning that they contain carbon and hydrogen bonded to each other. In addition to those two elements, they include nitrogen, oxygen, and, in a few cases, sulfur. The basic structure of an amino-acid molecule consists of a carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a fourth group that differs from one amino acid to another and often is referred to as the-R group or the side chain. The-R group, which can vary widely, is responsible for the differences in chemical properties.
This explanation sounds a bit technical and requires a background in chemistry that is beyond the scope of this essay, but let us simplify it somewhat. Imagine that the amino-acid molecule is like the face of a compass, with a carbon atom at the center. Raying out from the center, in the four directions of the compass, are lines representing chemical bonds to other atoms or groups of atoms. These directions are based on models that typically are used to represent amino-acid molecules, though north, south, east, and west, as used in the following illustration, are simply terms to make the molecule easier to visualize.
To the south of the carbon atom (C) is a hydrogen atom (H), which, like all the other atoms or groups, is joined to the carbon center by a chemical bond. To the north of the carbon center is what is known as an amino group (-NH2). The hyphen at the beginning indicates that such a group does not usually stand alone but normally is attached to some other atom or group. To the east is a carboxyl group, represented as-COOH. In the amino group, two hydrogen atoms are bonded to each other and then to nitrogen, whereas the carboxyl group has two separate oxygen atoms strung between a carbon atom and a hydrogen atom. Hence, they are not represented as O2.
Finally, off to the west is the R-group, which can vary widely. It is as though the other portions of the amino acid together formed a standard suffix in the English language, such as -tion. To the front of that suffix can be attached all sorts of terms drawn from root words, such as educate or
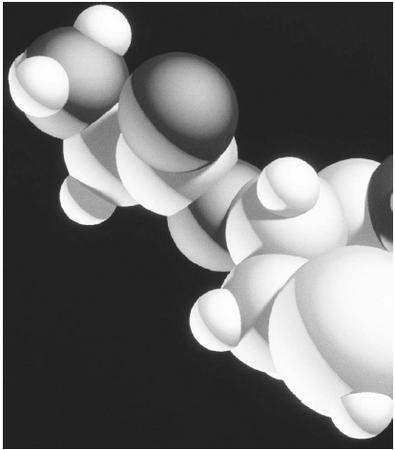
A FEW ADDITIONAL POINTS.
The name amino acid, in fact, comes from the amino group and the acid group, which are the most chemically reactive parts of the molecule. Each of the common amino acids has, in addition to its chemical name, a more familiar name and a three-letter abbreviation that frequently is used to identify it. In the present context, we are not concerned with these abbreviations. Amino-acid molecules, which contain an amino group and a carboxyl group, do not behave like typical molecules. Instead of melting at temperatures hotter than 392°F (200°C), they simply decompose. They are quite soluble, or capable of being dissolved, in water but are insoluble in nonpolar solvents (oil-and all oil-based products), such as benzene or ether.
RIGHT-HAND AND LEFT-HAND VERSIONS.
All of the amino acids in the human body, except glycine, are either right-hand or left-hand versions of the same molecule, meaning that in some amino acids the positions of the carboxyl group and the R-group are switched. Interestingly, nearly all of the amino acids occurring in nature are the left-hand versions of the molecules, or the L-forms. (There-fore, the model we have described is actually the left-hand model, though the distinctions between "right" and "left"—which involve the direction in which light is polarized—are too complex to discuss here.)
Right-hand versions (D-forms) are not found in the proteins of higher organisms, but they are present in some lower forms of life, such as in the cell walls of bacteria. They also are found in some antibiotics, among them, streptomycin, actinomycin, bacitracin, and tetracycline. These antibiotics, several of which are well known to the public at large, can kill bacterial cells by interfering with the formation of proteins necessary for maintaining life and for reproducing.
AMINO ACIDS AND PROTEINS
A chemical reaction that is characteristic of amino acids involves the formation of a bond, called a peptide linkage, between the carboxyl group of one amino acid and the amino group of a second amino acid. Very long chains of amino acids can bond together in this way to form proteins, which are the basic building blocks of all living things. The specific properties of each kind of protein are largely dependent on the kind and sequence of the amino acids in it. Other aspects of the chemical behavior of protein molecules are due to interactions between the amino and the carboxyl groups or between the various R-groups along the long chains of amino acids in the molecule.
NUMBERS AND COMBINATIONS.
Amino acids function as monomers, or individual units, that join together to form large, chainlike molecules called polymers, which may contain as few as two or as many as 3,000 amino-acid units. Groups of only two amino acids are called dipeptides, whereas three amino acids bonded together are called tripeptides. If there are more than 10 in a chain, they are termed polypeptides, and if there are 50 or more, these are known as proteins.
All the millions of different proteins in living things are formed by the bonding of only 20 amino acids to make up long polymer chains. Like the 26 letters of the alphabet that join together to form different words, depending on which letters are used and in which sequence, the 20 amino acids can join together in different combinations and series to form proteins. But whereas words usually have only about 10 or fewer letters, proteins typically are made from as few as 50 to as many as 3,000 amino acids. Because each amino acid can be used many times along the chain and because there are no restrictions on the length of the chain, the number of possible combinations for the formation of proteins is truly enormous. There are about two quadrillion different proteins that can exist if each of the 20 amino acids present in humans is used only once. Just as not all sequences of letters make sense, however, not all sequences of amino acids produce functioning proteins. Some other sequences can function and yet cause undesirable effects, as we shall see.
REAL-LIFE APPLICATIONS
DNA (deoxyribonucleic acid), a molecule in all cells that contains genetic codes for inheritance, creates encoded instructions for the synthesis of amino acids. In 1986, American medical scientist Thaddeus R. Dryja (1940-) used amino-acid sequences to identify and isolate the gene for a type of cancer known as retinoblastoma, a fact that illustrates the importance of amino acids in the body.
Amino acids are also present in hormones, chemicals that are essential to life. Among these hormones is insulin, which regulates sugar levels in the blood and without which a person would die. Another is adrenaline, which controls blood pressure and gives animals a sudden jolt of energy needed in a high-stress situation—running from a predator in the grasslands or (to a use a human example) facing a mugger in an alley or a bully on a playground. Biochemical studies of amino-acid sequences in hormones have made it
AMINO ACIDS AND NUTRITION
Just as proteins form when amino acids bond together in long chains, they can be broken down by a reaction called hydrolysis, the reverse of the formation of the peptide bond. That is exactly what happens in the process of digestion, when special digestive enzymes in the stomach enable the breaking down of the peptide linkage. (Enzymes are a type of protein—see Enzymes.) The amino acids, separated once again, are released into the small intestine, from whence they pass into the bloodstream and are carried throughout the organism. Each individual cell of the organism then can use these amino acids to assemble the new and different proteins required for its specific functions. Life thus is an ongoing cycle in which proteins are broken into individual amino-acid units, and new proteins are built up from these amino acids.
ESSENTIAL AMINO ACIDS.
Out of the many thousands of possible amino acids, humans require only 20 different kinds. Two others appear in the bodies of some animal species, and approximately 100 others can be found in plants. Considering the vast numbers of amino acids and possible combinations that exist in nature, the number of amino acids essential to life is extremely small. Yet of the 20 amino acids required by humans for making protein, only 12 can be produced within the body, whereas the other eight—isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine—must be obtained from the diet. (In addition, adults are capable of synthesizing arginine and histidine, but these amino acids are believed to be essential to growing children, meaning that children cannot produce them on their own.)
A complete protein is one that contains all of the essential amino acids in quantities sufficient for growth and repair of body tissue. Most proteins from animal sources, gelatin being the only exception, contain all the essential amino acids and are therefore considered complete proteins. On the other hand, many plant proteins do not contain all of the essential amino acids. For example, lysine is absent from corn, rice, and wheat, whereas corn also lacks tryptophan and rice lacks threonine. Soybeans are lacking in methionine. Vegans, or vegetarians who consume no animal proteins in their diets (i.e., no eggs, dairy products, or the like) are at risk of malnutrition, because they may fail to assimilate one or more essential amino acid.
AMINO ACIDS, HEALTH, AND DISEASE
Amino acids can be used as treatments for all sorts of medical conditions. For example, tyrosine may be employed in the treatment of Alzheimer's disease, a condition characterized by the onset of dementia, or mental deterioration, as well as for alcohol-withdrawal symptoms. Taurine is administered to control epileptic seizures, treat high blood pressure and diabetes, and support the functioning of the liver. Numerous other amino acids are used in treating a wide array of other diseases. Sometimes the disease itself involves a problem with amino-acid production or functioning. In the essay Vitamins, there is a discussion of pellagra, a disease resulting from a deficiency of the B-group vitamin known as niacin. Pellagra results from a diet heavy in corn, which, as we have noted, lacks lysine and tryptophan. Its symptoms often are described as the "three Ds": diarrhea, dermatitis (or skin inflammation), and dementia. Thanks to a greater understanding of nutrition and health, pellagra has been largely eradicated, but there still exists a condition with almost identical symptoms: Hartnup disease, a genetic disorder named for a British family in the late 1950s who suffered from it.
Hartnup disease is characterized by an inability to transport amino acids from the kidneys to the rest of the body. The symptoms at first seemed to suggest to physicians that the disease, which is present in one of about 26,000 live births, was pellagra. Tests showed that sufferers did not have inadequate tryptophan levels, however, as would have been the case with pellagra. On the other hand, some 14 amino acids have been found in excess within the urine of Hartnup disease sufferers, indicating that rather than properly transporting amino acids, their bodies are simply excreting them. This is a potentially very serious condition, but it can be treated with the B vitamin nicotinamide, also used to treat pellagra. Supplementation of tryptophan in the diet also has shown positive results with some patients.
SICKLE CELL ANEMIA.
It is also possible for small mistakes to occur in the amino-acid sequence within the body. While these mistakes sometimes can be tolerated in nature without serious problems, at other times a single misplaced amino acid in the polymer chain can bring about an extremely serious condition of protein malfunctioning. An example of this is sickle cell anemia, a fatal disease ultimately caused by a single mistake in the amino acid sequence. In the bodies of sickle cell anemia sufferers, who are typically natives of sub-Saharan Africa or their descendants in the United States or elsewhere, glutamic acid is replaced by valine at the sixth position from the end of the protein chain in the hemoglobin molecule. (Hemoglobin is an iron-containing pigment in red blood cells that is responsible for transporting oxygen to the tissues and removing carbon dioxide from them.) This small difference makes sickle cell hemoglobin molecules extremely sensitive to oxygen deficiencies. As a result, when the red blood cells release their oxygen to the tissues, as all red blood cells do, they fail to re-oxygenate in a normal fashion and instead twist into the shape that gives sickle cell anemia its name. This causes obstruction of the blood vessels. Before the development of a treatment with the drug hydroxyurea in the mid-1990s, the average life expectancy of a person with sickle cell anemia was about 45 years.
AMINO ACIDS AND THE DISTANT PAST
The Evolution essay discusses several types of dating, a term referring to scientific efforts directed toward finding the age of a particular item or phenomenon. Methods of dating are either relative (i.e., comparative and usually based on rock strata, or layers) or absolute. Whereas relative dating does not involve actual estimates of age in years, absolute dating does. One of the first types of absolute-dating techniques developed was amino-acid racimization, introduced in the 1960s. As noted earlier, there are "left-hand" L-forms and "right-hand" D-forms of all amino acids. Virtually all living organisms (except some microbes) incorporate only the L-forms, but once the organism dies, the L-amino acids gradually convert to the mirror-image D-amino acids.
Numerous factors influence the rate of conversion, and though amino-acid racimization was popular as a form of dating in the 1970s, there are problems with it. For instance, the process occurs at different rates for different amino acids, and the rates are further affected by such factors as moisture and temperature. Because of the uncertainties with amino-acid racimization, it has been largely replaced by other absolute-dating methods, such as the use of radioactive isotopes.
Certainly, amino acids themselves have offered important keys to understanding the planet's distant past. The discovery, in 1967 and 1968, of sedimentary rocks bearing traces of amino acids as much as three billion years old had an enormous impact on the study of Earth's biological history. Here, for the first time, was concrete evidence of life—at least, in a very simple chemical form—existing billions of years before the first true organism. The discovery of these amino-acid samples greatly influenced scientists' thinking about evolution, particularly the very early stages in which the chemical foundations of life were established.
WHERE TO LEARN MORE
"Amino Acids." Institute of Chemistry, Department of Biology, Chemistry, and Pharmacy, Freie Universität, Berlin (Web site). <http://www.chemie.fu-berlin.de/chemistry/bio/amino-acids_en.html>.
Goodsell, David S. Our Molecular Nature: The Body's Motors, Machines, and Messages. New York: Copernicus, 1996.
"Introduction to Amino Acids." Department of Crystallography, Birbeck College (Web site). <http://www.cryst.bbk.ac.uk/education/AminoAcid/overview.html>.
Michal, Gerhard. Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology. New York: John Wiley and Sons, 1999.
Newstrom, Harvey. Nutrients Catalog: Vitamins, Minerals, Amino Acids, Macronutrients—Beneficial Use, Helpers, Inhibitors, Food Sources, Intake Recommendations, and Symptoms of Over or Under Use. Jefferson, NC: McFarland and Company, 1993.
Ornstein, Robert E., and Charles Swencionis. The Healing Brain: A Scientific Reader. New York: Guilford Press, 1990.
Reference Guide for Amino Acids (Web site). <http://www.realtime.net/anr/aminoacd.html#tryptophn>.
Silverstein, Alvin, Virginia B. Silverstein, and Robert A. Silverstein. Proteins. Illus. Anne Canevari Green. Brookfield, CT: Millbrook Press, 1992.
Springer Link: Amino Acids (Web site). <http://link.springer.de/link/service/journals/00726/>.
KEY TERMS
AMINO ACIDS:
Organic compounds made of carbon, hydrogen, oxygen, nitro gen, and (in some cases) sulfur bonded in characteristic formations. Strings of amino acids make up proteins.
AMINO GROUP:
The chemical forma tion NH2, which is part of all amino acids.
BIOCHEMISTRY:
The area of the bio logical sciences concerned with the chemical substances and processes in organisms.
CARBOXYL GROUP:
The formation COOH, which is common to all amino acids.
COMPOUND:
A substance in which atoms of more than one element are bond ed chemically to one another.
DIPEPTIDE:
A group of only two amino acids.
DNA:
Deoxyribonucleic acid, a molecule in all cells and many viruses containing genetic codes for inheritance.
ENZYME:
A protein material that speeds up chemical reactions in the bodies of plants and animals.
ESSENTIAL AMINO ACIDS:
Amino acids that cannot be manufactured by the body, and which therefore must be obtained from the diet. Proteins that contain essential amino acids are known as complete proteins.
GENE:
A unit of information about a particular heritable (capable of being inherited) trait that is passed from parent to offspring, stored in DNA molecules called chromosomes.
HORMONE:
Molecules produced by living cells, which send signals to spots remote from their point of origin and induce specific effects on the activities of other cells.
MOLECULE:
A group of atoms, usually but not always representing more than one element, joined in a structure. Compounds typically are made up of molecules.
ORGANIC:
At one time chemists used the term organic only in reference to living things. Now the word is applied to compounds containing carbon and hydrogen.
PEPTIDE LINKAGE:
A bond between the carboxyl group of one amino acid and the amino group of a second amino acid.
POLYMERS:
Large, chainlike molecules composed of numerous subunits known as monomers.
POLYPEPTIDE:
A group of between 10 and 50 amino acids.
PROTEINS:
Large molecules built from long chains of 50 or more amino acids. Proteins serve the functions of promoting normal growth, repairing damaged tissue, contributing to the body's immune system, and making enzymes.
RNA:
Ribonucleic acid, the molecule translated from DNA in the cell nucleus, the control center of the cell, that directs protein synthesis in the cytoplasm, or the space between cells.
SYNTHESIZE:
To manufacture chemically, as in the body.
TRIPEPTIDE:
A group of three amino acids.
User Contributions:
Comment about this article, ask questions, or add new information about this topic:
Amino Acids forum
Please mail me similar subject(s)/reports,scientific advancement, etc.
Keep it up. Bye.