Minerals - How it works
Introduction to Minerals
The particulars of the mineral definition deserve some expansion, especially inasmuch as mineral has an everyday definition somewhat broader than its scientific definition. In everyday usage, minerals would be the natural, nonliving materials that make up rocks and are mined from the earth. According to this definition, minerals would include all metals, gemstones, clays, and ores. The scientific definition, on the other hand, is much narrower, as we shall see.
The fact that a mineral must be inorganic brings up another term that has a broader meaning in everyday life than in the world of science. At one time, the scientific definition of organic was more or less like the meaning assigned to it by nonscientists today, as describing all living or formerly living things, their parts, and substances that come from them. Today, however, chemists use the word organic to refer to any compound that contains carbon bonded to hydrogen, thus excluding carbonates (which are a type of mineral) and oxides such as carbon dioxide or carbon monoxide. Because a mineral must be inorganic, this definition eliminates coal and peat, both of which come from a wide-ranging group of organic substances known as hydrocarbons.
A mineral also occurs naturally, meaning that even though there are artificial substances that might be described as "mineral-like," they are not minerals. In this sense, the definition of a mineral is even more restricted than that of an element, discussed later in this essay, even though there are nearly 4,000 minerals and more than 92 elements. The number 92, of course, is not arbitrary: that is the number of elements that occur in nature. But there are additional elements, numbering 20 at the end of the twentieth century, that have been created artificially.
PHYSICAL AND CHEMICAL PROPERTIES OF MINERALS.
The specific characteristics of minerals can be discussed both in physical and in chemical terms. From the standpoint of physics, which is concerned with matter, energy, and the interactions between the two, minerals would be described as crystalline solids. The definition of a mineral is narrowed further in terms of its chemistry, or its atomic characteristics, since a mineral must be of unvarying composition.
A mineral, then, must be solid under ordinary conditions of pressure and temperature. This excludes petroleum, for instance (which, in any case, would have been disqualified owing to its organic origins), as well as all other liquids and gases. Moreover, a mineral cannot be just any type of solid but must be a crystalline one—that is, a solid in which the constituent parts have a simple and definite geometric arrangement that is repeated in all directions. This rule, for instance, eliminates clay, an example of an amorphous solid.
Chemically, a mineral must be of unvarying composition, a stipulation that effectively limits minerals to elements and compounds. Neither sand nor glass, for instance, is a mineral, because the composition of both can vary. Another way of putting this is to say that all minerals must have a definite chemical formula, which is not true of sand, dirt, glass, or any other mixture. Let us now look a bit more deeply into the nature of elements and compounds, which are collectively known as pure substances, so as to understand the minerals that are a subset of this larger grouping.
Elements
The periodic table of elements is a chart that appears in most classrooms where any of the physical sciences are taught. It lists all elements in order of atomic number, or the number of protons (positively charged subatomic particles) in the atomic nucleus. The highest atomic number of any naturally occurring element is 92, for uranium, though it should be noted that a very few elements with an atomic number lower than 92 have never actually been found on Earth. On the other hand, all elements with an atomic number higher than 92 are artificial, created either in laboratories or as the result of atomic testing.
An element is a substance made of only one type of atom, meaning that it cannot be broken down chemically to create a simpler substance. In the sense that each is a fundamental building block in the chemistry of the universe, all elements are, as it were, "created equal." They are not equal, however, in terms of their abundance. The first two elements on the periodic table, hydrogen and helium, represent 99.9% of the matter in the entire universe. Though Earth contains little of either, our planet is only a tiny dot within the vastness of space; by contrast, stars such as our Sun are composed almost entirely of those elements (see Sun, Moon, and Earth).
ABUNDANCE ON EARTH.
Of all elements, oxygen is by far the most plentiful on Earth, representing nearly half—49.2%—of the total mass of atoms found on this planet. (Here the term mass refers to the known elemental mass of the planet's atmosphere, waters, and crust; below the crust, scientists can only speculate, though it is likely that much of Earth's interior consists of iron.)
Together with silicon (25.7%), oxygen accounts for almost exactly three-fourths of the elemental mass of Earth. If we add in aluminum (7.5%), iron (4.71%), calcium (3.39%), sodium (2.63%), potassium (2.4%), and magnesium (1.93%), these eight elements make up about 97.46% of Earth's material. Hydrogen, so plentiful in the universe at large, ranks ninth on Earth, accounting for only 0.87% of the planet's known elemental mass. Nine other elements account for a total of 2% of Earth's composition: titanium (0.58%), chlorine (0.19%), phosphorus (0.11%), manganese (0.09%), carbon (0.08%), sulfur (0.06%), barium (0.04%), nitrogen (0.03%), and fluorine (0.03%). The remaining 0.49% is made up of various other elements.
Looking only at Earth's crust, the numbers change somewhat, especially at the lower end of the list. Listed below are the 12 most abundant elements in the planet's crust, known to earth scientists simply as "the abundant elements." These 12, which make up 99.23% of the known crustal mass, together form approximately 40 different minerals that account for the vast majority of that 99.23%. Following the name and chemical symbol of each element is the percentage of the crustal mass it composes.
Abundance of Elements in Earth's Crust
- Oxygen (O): 45.2%
- Silicon (Si): 27.2%
- Aluminum (Al): 8.0%
- Iron (Fe): 5.8%
- Calcium (Ca): 5.06%
- Magnesium (Mg): 2.77%
- Sodium (Na): 2.32%
- Potassium (K): 1.68%
- Titanium (Ti): 0.86%
- Hydrogen (H): 0.14%
- Manganese (Mn): 0.1%
- Phosphorus (P): 0.1%
Atoms, Molecules, and Bonding
As noted earlier, an element is identified by the number of protons in its nucleus, such that any atom with six protons must be carbon, since carbon has an atomic number of 6. The number of electrons, or negatively charged subatomic particles, is the same as the number of protons, giving an atom no net electric charge.
An atom may lose or gain electrons, however, in which case it becomes an ion, an atom or group of atoms with a net electric charge. An atom that has gained electrons, and thus has a negative charge, is called an anion. On the other hand, an atom that has lost electrons, thus becoming positive in charge, is a cation.
In addition to protons and electrons, an atom has neutrons, or neutrally charged particles, in its nucleus. Neutrons have a mass close to that of a proton, which is much larger than that of an electron, and thus the number of neutrons in an atom has a significant effect on its mass. Atoms that have the same number of protons (and therefore are of the same element), but differ in their number of neutrons, are called isotopes.
COMPOUNDS AND MIXTURES.
Whereas there are only a very few elements, there are millions of compounds, or substances made of more than one atom. A simple example is water, formed by the bonding of two hydrogen atoms with one oxygen atom; hence the chemical formula for water, which is H 2 O. Note that this is quite different from a mere mixture of hydrogen and oxygen, which would be something else entirely. Given the gaseous composition of the two elements, combined with the fact that both are extremely flammable, the result could hardly be more different from liquid water, which, of course, is used for putting out fires.
The difference between water and the hydrogen-oxygen mixture described is that whereas the latter is the result of mere physical mixing, water is created by chemical bonding. Chemical bonding is the joining, through electromagnetic attraction, of two or more atoms to create a compound. Of the three principal subatomic particles, only electrons are involved in chemical bonding—and only a small portion of those, known as valence electrons, which occupy the outer shell of an atom. Each element has a characteristic pattern of valence electrons, which determines the ways in which the atom bonds.
CHEMICAL BONDING.
Noble gases, of which helium is an example, are noted for their lack of chemical reactivity, or their resistance to bonding. While studying these elements, the German chemist Richard Abegg (1869-1910) discovered that they all have eight valence electrons. His observation led to one of the most important principles of chemical bonding: atoms bond in such a way that they achieve the electron configuration of a noble gas. This concept, known as the octet rule, has been shown to be the case in most stable chemical compounds.
Abegg hypothesized that atoms combine with one another because they exchange electrons in such a way that both end up with eight valence electrons. This was an early model of ionic bonding, which results from attractions between ions with opposite electric charges: when they bond, these ions "complete" each other. Metals tend to form cations and bond with nonmetals that have formed anions. The bond between anions and cations is known as an ionic bond, and is extremely strong.
The other principal type of bond is a covalent bond. The result, once again, is eight valence electrons for each atom, but in this case, the nuclei of the two atoms share electrons. Neither atom "owns" them; rather, they share electrons. Today, chemists understand that most bonds are neither purely ionic nor purely covalent; instead, there is a wide range of hybrids between the two extremes, which are a function of the respective elements' electronegativity, or the relative ability of an atom to attract valence electrons. If one element has a much higher electronegativity value than the other one, the bond will be purely ionic, but if two elements have equal electronegativity values, the bond is purely covalent. Most bonds, however, fall somewhere between these two extremes.
INTERMOLECULAR BONDING.
Chemical bonds exist between atoms and within a molecule. But there are also bonds between molecules, which affect the physical composition of a substance. The strength of intermolecular bonds is affected by the characteristics of the interatomic, or chemical, bond.
For example, the difference in electronegativity values between hydrogen and oxygen is great enough that the bond between them is not purely covalent, but instead is described as a polar covalent bond. Oxygen has a much higher electronegativity (3.5) than hydrogen (2.1), and therefore the electrons tend to gravitate toward the oxygen atom. As a result, water molecules have a strong negative charge on the side occupied by the oxygen atom, with a resulting positive charge on the hydrogen side.
By contrast, molecules of petroleum, a combination of carbon and hydrogen, tend to be nonpolar, because carbon (with an electronegativity value of 2.5) and hydrogen have very similar electronegativity values. Therefore the electric charges are more or less evenly distributed in the molecule. As a result, water molecules form strong attractions, known as dipole-dipole attractions, to each other. Molecules of petroleum, on the other hand, have little attraction to each other, and the differences in charge distribution account for the fact that water and oil do not mix.
Even weaker than the bonds between non-polar molecules, however, are those between highly reactive elements, such as the noble gases and the "noble metals"—gold, silver, and copper, which resist bonding with other elements. The type of intermolecular attraction that exists in such a situation is described by the term London dispersion forces, a reference to the German-born American physicist Fritz Wolfgang London (1900-1954).
The bonding between molecules of most other metals, however, is described by the electron sea model, which depicts metal atoms as floating in a "sea" of valence electrons. These valence electrons are highly mobile within the crystalline structure of the metal, and this mobility helps explain metals' high electric conductivity. The ease with which metal crystals allow themselves to be rearranged explains not only metals' ductility (their ability to be shaped) but also their ability to form alloys, a mixture containing two or more metals.
The Crystalline Structure of Minerals
By definition, a solid is a type of matter whose particles resist attempts at compression. Because of their close proximity, solid particles are fixed in an orderly and definite pattern. Within the larger category of solids are crystalline solids, or those in which the constituent parts are arranged in a simple, definite geometric pattern that is repeated in all directions.
The term crystal is popularly associated with glass and with quartz, but only one of these is a crystalline solid. Quartz is a member of the silicates, a large group of minerals that we will discuss later in this essay. Glass, on the other hand, is an amorphous solid, meaning that its molecules are not arranged in an orderly pattern.
CRYSTAL SYSTEMS.
Elsewhere in this book (Earth, Science, and Nonscience and Planetary Science), there is considerable discussion of misconceptions originating with Aristotle (384-322 B.C. ). Despite his many achievements, including significant contributions to the biological sciences, the great Greek philosopher spawned a number of erroneous concepts, which prevailed in the physical sciences until the dawn of the modern era. At least Aristotle made an attempt at scientific study, however; for instance, he dissected dead animals to observe their anatomic structures. His teacher, Plato (427?-347 B.C. ), on the other hand, is hardly ever placed among the ranks of those who contributed, even ever so slightly, to progress in the sciences.
There is a reason for this. Plato, in contrast to his pupil, made virtually no attempt to draw his ideas about the universe from an actual study of it. Within Plato's worldview, the specific qualities of any item, including those in the physical world, reflected the existence of perfect and pure ideas that were more "real" than the physical objects themselves. Typical of his philosophy was his idea of the five Platonic solids, or "perfect" geometric shapes that, he claimed, formed the atomic substructure of the world.
The "perfection" of the Platonic solids lay in the fact that they are the only five three-dimensional objects in which the faces constitute a single type of polygon (a closed shape with three or more sides, all straight), while the vertices (edges) are all alike. These five are the tetrahedron, octahedron, and icosahedron, composed of equilateral triangles (four, eight, and twenty, respectively); the cube, which, of course, is made of six squares; and the dodecahedron, made up of twelve pentagons. Plato associated the latter solid with the shape of atoms in outer space, while the other four corresponded to what the Greeks believed were the elements on Earth: fire (tetrahedron), earth (cube), air (octahedron), and water (icosahedron).
All of this, of course, is nonsense from the standpoint of science, though the Platonic solids are of interest within the realm of mathematics. Yet amazingly, Plato in his unscientific way actually touched on something close to the truth, as applied to the crystalline structure of minerals.
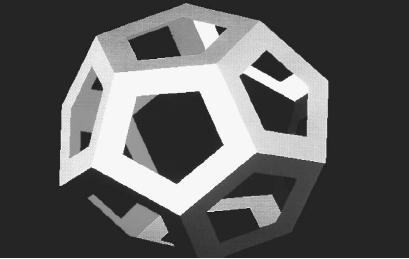
An isometric crystal system is the most symmetrical of all, with faces and angles that are most clearly uniform. Because of differing types of polygon that make up the faces, as well as differing numbers of vertices, these crystals appear in 15 forms, several of which are almost eerily reminiscent of Plato's solids: not just the cube (exemplified by halite crystals) but also the octahedron (typical of spinels) and even the dodecahedron (garnets).
Comment about this article, ask questions, or add new information about this topic: