Gas Laws - Real-life applications

Pressure Changes
OPENING A SODA CAN.
Inside a can or bottle of carbonated soda is carbon dioxide gas (CO 2 ), most of which is dissolved in the drink itself. But some of it is in the space (sometimes referred to as "head space") that makes up the difference between the volume of the soft drink and the volume of the container.
At the bottling plant, the soda manufacturer adds high-pressure carbon dioxide to the head space in order to ensure that more CO 2 will be absorbed into the soda itself. This is in accordance with Henry's law: the amount of gas (in this case CO 2 ) dissolved in the liquid (soda) is directly proportional to the partial pressure of the gas above the surface of the solution—that is, the CO 2 in the head space. The higher the pressure of the CO 2 in the head space, the greater the amount of CO 2 in the drink itself; and the greater the CO 2 in the drink, the greater the "fizz" of the soda.
Once the container is opened, the pressure in the head space drops dramatically. Once again, Henry's law indicates that this drop in pressure will be reflected by a corresponding drop in the amount of CO 2 dissolved in the soda. Over a period of time, the soda will release that gas, and will eventually go "flat."
FIRE EXTINGUISHERS.
A fire extinguisher consists of a long cylinder with an operating lever at the top. Inside the cylinder is a tube of carbon dioxide surrounded by a quantity of water, which creates pressure around the CO 2 tube. A siphon tube runs vertically along the length of the extinguisher, with one opening near the bottom of the water. The other end opens in a chamber containing a spring mechanism attached to a release valve in the CO 2 tube.
The water and the CO 2 do not fill the entire cylinder: as with the soda can, there is "head space," an area filled with air. When the operating lever is depressed, it activates the spring mechanism, which pierces the release valve at the top of the CO 2 tube. When the valve opens, the CO 2 spills out in the "head space," exerting pressure on the water. This high-pressure mixture of water and carbon dioxide goes rushing out of the siphon tube, which was opened when the release valve was depressed. All of this happens, of course, in a fraction of a second—plenty of time to put out the fire.
AEROSOL CANS.
Aerosol cans are similar in structure to fire extinguishers, though with one important difference. As with the fire extinguisher, an aerosol can includes a nozzle that depresses a spring mechanism, which in turn allows fluid to escape through a tube. But instead of a gas cartridge surrounded by water, most of the can's interior is made up of the product (for instance, deodorant), mixed with a liquid propellant.
The "head space" of the aerosol can is filled with highly pressurized propellant in gas form, and in accordance with Henry's law, a corresponding proportion of this propellant is dissolved in the product itself. When the nozzle is depressed, the pressure of the propellant forces the product out through the nozzle.
A propellant, as its name implies, propels the product itself through the spray nozzle when the latter is depressed. In the past, chlorofluorocarbons (CFCs)—manufactured compounds containing carbon, chlorine, and fluorine atoms—were the most widely used form of propellant. Concerns over the harmful effects of CFCs on the environment, however, has led to the development of alternative propellants, most notably hydrochlorofluorocarbons (HCFCs), CFC-like compounds that also contain hydrogen atoms.
When the Temperature Changes
A number of interesting things, some of them unfortunate and some potentially lethal, occur when gases experience a change in temperature. In these instances, it is possible to see the gas laws—particularly Boyle's and Charles's—at work.
There are a number of examples of the disastrous effects that result from an increase in the temperature of a product containing combustible gases, as with natural gas and petroleum-based products. In addition, the pressure on the gases in aerosol cans makes the cans highly explosive—so much so that discarded cans at a city dump may explode on a hot summer day. Yet there are other instances when heating a gas can produce positive effects.
A hot-air balloon, for instance, floats because the air inside it is not as dense than the air outside. By itself, this fact does not depend on any of the gas laws, but rather reflects the concept of buoyancy. However, the way in which the density of the air in the balloon is reduced does indeed reflect the gas laws.
According to Charles's law, heating a gas will increase its volume. Also, as noted in the first and second propositions regarding the behavior of gases, gas molecules are highly nonattractive to one another, and therefore, there is a great deal of space between them. The increase in volume makes that space even greater, leading to a significant difference in density between the air in the balloon and the air outside. As a result, the balloon floats, or becomes buoyant.
Although heating a gas can be beneficial, cooling a gas is not always a wise idea. If someone were to put a bag of potato chips into a freezer, thinking this would preserve their flavor, he would be in for a disappointment. Much of what maintains the flavor of the chips is the pressurization of the bag, which ensures a consistent internal environment in which preservative chemicals, added during the manufacture of the chips, can keep them fresh. Placing the bag in the freezer causes a reduction in pressure, as per Gay-Lussac's law, and the bag ends up a limp version of its earlier self.
Propane tanks and tires offer an example of the pitfalls that may occur by either allowing a gas to heat up or cool down by too much. Because most propane tanks are made according to strict regulations, they are generally safe, but it is not entirely inconceivable that an extremely hot summer day could cause a defective tank to burst. Certainly the laws of physics are there: an increase in temperature leads to an increase in pressure, in accordance with Gay-Lussac's law, and could lead to an explosion.
Because of the connection between heat and pressure, propane trucks on the highways during the summer are subjected to weight tests to ensure that they are not carrying too much of the gas. On the other hand, a drastic reduction in temperature could result in a loss in gas pressure. If a propane tank from Florida were transported by truck during the winter to northern Canada, the pressure would be dramatically reduced by the time it reached its destination.
Gas Reactions That Move and Stop a Car
In operating a car, we experience two examples of gas laws in operation. One of these, common to everyone, is that which makes the car run: the combustion of gases in the engine. The other is, fortunately, a less frequent phenomenon—but it can and does save lives. This is the operation of an air bag, which, though it is partly related to laws of motion, depends also on the behaviors explained in Charles's law.
With regard to the engine, when the driver pushes down on the accelerator, this activates a throttle valve that sprays droplets of gasoline mixed with air into the engine. (Older vehicles used a carburetor to mix the gasoline and air, but most modern cars use fuel-injection, which sprays the air-gas combination without requiring an intermediate step.) The mixture goes into the
While the mixture is still compressed (high pressure, high density), an electric spark plug produces a flash that ignites it. The heat from this controlled explosion increases the volume of air, which forces the piston down into the cylinder. This opens an outlet valve, causing the piston to rise and release exhaust gases.
As the piston moves back down again, an inlet valve opens, bringing another burst of gasoline-air mixture into the chamber. The piston, whose downward stroke closed the inlet valve, now shoots back up, compressing the gas and air to repeat the cycle. The reactions of the gasoline and air are what move the piston, which turns a crankshaft that causes the wheels to rotate.
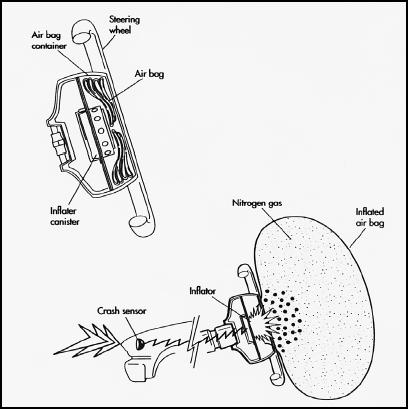
So much for moving—what about stopping? Most modern cars are equipped with an airbag, which reacts to sudden impact by inflating. This protects the driver and front-seat passenger, who, even if they are wearing seatbelts, may otherwise be thrown against the steering wheel or dashboard..
But an airbag is much more complicated than it seems. In order for it to save lives, it must deploy within 40 milliseconds (0.04 seconds). Not only that, but it has to begin deflating before the body hits it. An airbag does not inflate if a car simply goes over a bump; it only operates in situations when the vehicle experiences extremedeceleration. When this occurs, there is a rapidtransfer of kinetic energy to rest energy, as with the earlier illustration of a stone hitting concrete. And indeed, if you were to smash against a fullyinflated airbag, it would feel like hitting concrete—with all the expected results.
The airbag's sensor contains a steel ballattached to a permanent magnet or a stiff spring. The spring holds it in place through minormishaps in which an airbag would not be warranted—for instance, if a car were simply to be "tapped" by another in a parking lot. But in a case of sudden deceleration, the magnet or springreleases the ball, sending it down a smooth bore. It flips a switch, turning on an electrical circuit.This in turn ignites a pellet of sodium azide, which fills the bag with nitrogen gas.
The events described in the above illustration take place within 40 milliseconds—less time than it takes for your body to come flying forward; and then the airbag has to begin deflating before the body reaches it. At this point, the highly pressurized nitrogen gas molecules begin escaping through vents. Thus as your body hits the bag, the deflation of the latter is moving it in the same direction that your body is going—only much, much more slowly. Two seconds after impact, which is an eternity in terms of the processes involved, the pressure inside the bag has returned to 1 atm.
WHERE TO LEARN MORE
Beiser, Arthur. Physics, 5th ed. Reading, MA: Addison-Wesley, 1991.
"Chemistry Units: Gas Laws." (Web site). <http://bio.bio.rpi.edu/MS99/ausemaW/chem/gases.hmtl> (February 21, 2001).
Laws of Gases. New York: Arno Press, 1981.
Macaulay, David. The New Way Things Work. Boston: Houghton Mifflin, 1998.
Mebane, Robert C. and Thomas R. Rybolt. Air and Other Gases. Illustrations by Anni Matsick. New York: Twenty-First Century Books, 1995.
"Tutorials—6." <http://www.chemistrycoach.com/tutorials-6.html> (February 21, 2001).
Comment about this article, ask questions, or add new information about this topic: