Atom
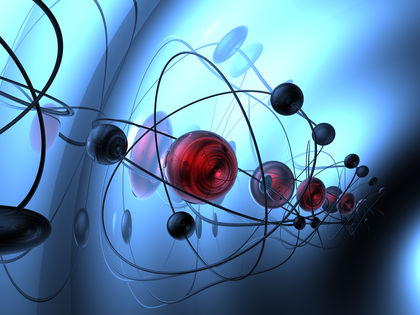
An atom is the smallest particle of a element that has all the properties of that element. Imagine that you decide to cut a chunk of aluminum metal into half, over and over again. At some point, you would need very small tools to do the cutting, tools smaller than anything that really exists. However, you would eventually get to the very smallest piece of aluminum that still has all the properties of the original chunk. That smallest piece is an atom of aluminum.
History
One of the questions that ancient Greeks thinkers debated was the structure of matter. Is matter, they asked, continuous or discontinuous? That is, in the aluminum example mentioned above, can a person continue to cut a chunk of aluminum into smaller pieces for ever and ever? Or would the person eventually reach some smallest piece of aluminum that could be divided no further?
Two of the philosophers who argued for the latter opinion were Leucippus (born about 490 B.C. ) and his student Democritus (c. 470–c. 380 B.C. ). It was Democritus, in fact, who first used the word atomos to describe the smallest possible particles of matter. Atomos means "indivisible" in Greek.
The particle theory of matter was not developed to any great extent for more than 2,000 years. Then, in 1808, English chemist John Dalton (1766–1844) rephrased the theory in modern terms. Dalton thought of atoms as tiny, indivisible particles, similar to ball bearings or marbles. Dalton's theory of atoms satisfactorily explained what was then known about matter; it was quickly accepted by many other (although not all) chemists.
In the two centuries since Dalton first proposed the modern concept of atoms, that concept has undergone some dramatic changes. We no longer believe that atoms are indivisible particles. We know that they consist of smaller units, known as protons, neutrons, and electrons. These particles are called subatomic particles because they are all smaller than an atom itself. Some subatomic particles are capable of being divided into even smaller units known as quarks.
Modern models of the atom
Scientists think of atoms today in mathematical terms. They use mathematical equations to represent the likelihood of finding electrons in various parts of the atom and to describe the structure of the atomic nucleus, in which protons and neutrons exist.
Most people still find it helpful to think about atoms in physical terms that we can picture in our minds. For most purposes, these pictures are good enough to understand what atoms are like. An atom consists of two parts, a nucleus and a set of one or more electrons spinning around the nucleus.
The nucleus is located at the center of an atom. It consists of one or more protons and, with the exception of the hydrogen atom, one or more neutrons. The number of protons in an atom is given the name atomic number. An atom with one proton in its nucleus has an atomic number of 1, while an atom with sixteen protons in its nucleus has an atomic number of 16. The total number of protons and neutrons in a nucleus is called the atom's mass number. An atom with two protons and two neutrons, for example, has a mass number of 4.
The number of electrons located outside the nucleus of an atom is always the same as the number of protons. An atom with seven protons in its nucleus (no matter how many neutrons) also has seven electrons outside the nucleus. Those electrons travel in paths around the nucleus somewhat similar to the orbits followed by planets around the Sun. Each
of these orbits can hold a certain number of electrons. The first orbit, for example, may hold up to two electrons, but no more. The second orbit may hold up to eight electrons, but no more. The third orbit may hold a maximum of 18 electrons.
These limits determine how the electrons in an atom are distributed. Suppose that the nucleus of an atom contains nine protons. Then the atom also contains nine electrons outside the nucleus. Two of the electrons can be in the first orbit around the nucleus, but the other seven must go to the second orbit.
The term electron orbit is not really correct, even if it does help understand what an electron's path looks like. A better term is electron energy level. The closer an electron is to the nucleus of an atom, the less energy it has; the farther away from the nucleus, the more energy it has.
Physical dimensions
An atom and the particles of which it is composed can be fully described by knowing three properties: mass, electrical charge, and spin.
The mass of protons, neutrons, and electrons is so small that normal units of measurement (such as the gram or centigram) are not used. As an example, the actual mass of a proton is 1.6753 × 10 −24 g, or 0.000 000 000 000 000 000 000 001 675 3 grams. Numbers of this size are so inconvenient to work with that scientists have invented a special unit known as the atomic mass unit (abbreviation: amu) to state the mass of subatomic particles. One atomic mass unit (1 amu) is approximately equal to the mass of a single proton. Using this measure, the mass of a neutron is also about 1 amu, and the mass of an electron, about 0.00055 amu.
The mass of an atom, then, is equal to the total mass of all protons, neutrons, and electrons added together. In the case of the oxygen atom, that mass is expressed as follows:
mass of oxygen atom = mass of 8 protons + 8 neutrons + 8 electrons
mass of one oxygen atom = 8 amu + 8 amu + (8 × 0.00055 amu)
mass of one oxygen atom = 16.0044 amu
The total mass of an atom is called its atomic mass or, less accurately, its atomic weight. As you can see, the mass of an atom depends primarily on the mass of its protons and neutrons and is hardly affected by the mass of its electrons.
The actual mass and size of atoms using ordinary units of measurement are both very small. The mass of one oxygen atom measured in grams is 5.36 × 10 −23 g or 0.000 000 000 000 000 000 000 053 6 grams.
The dimensions of an atom and its nucleus are also amazingly small. The distance across the outside of a typical atom is about 10 −10 m, or 0.000 000 000 1 meters. In contrast, the distance across a nucleus is about 10 −15 m, or 0.000 000 000 000 001 meters. In another words, an atom is about 100,000 times larger in size than it its nucleus. To get some idea of this comparison, imagine a pea placed in the center of a large football stadium. If the pea represents the nucleus of an atom, the closest electrons in the atom would be spinning around outside the outermost reaches of the stadium's upper seats.
[ See also Atomic mass ; Atomic theory ; Electron ; Element, chemical ; Matter, states of ; Subatomic particles ]