Nuclear fission

Nuclear fission is a process in which the nucleus of a heavy atom is broken apart into two or more smaller nuclei. The reaction was first discovered in the late 1930s when a target of uranium metal was bombarded with neutrons. Uranium nuclei broke into two smaller nuclei of roughly equal size with the emission of very large amounts of energy. Some scientists immediately recognized the potential of the nuclear fission reaction for the production of bombs and other types of weapons as well as for the generation of power for peacetime uses.
History
The fission reaction was discovered accidentally in 1938 by two German physicists, Otto Hahn (1879–1968) and Fritz Strassmann (1902–1980). Hahn and Strassmann had been doing a series of experiments in which they used neutrons to bombard various elements. When they bombarded copper, for example, a radioactive form of copper was produced. Other elements became radioactive in the same way.
Their work with uranium, however, produced entirely different results. In fact, the results were so unexpected that Hahn and Strassmann were unable to offer a satisfactory explanation for what they observed. That explanation was provided, instead, by German physicist Lise Meitner (1878–1968) and her nephew Otto Frisch (1904–1979). Meitner was a longtime colleague of Hahn who had left Germany due to anti-Jewish persecution.
In most nuclear reactions, an atom changes from a stable form to a radioactive form, or it changes to a slightly heavier or a slightly lighter atom. Copper (element number 29), for example, might change from a stable form to a radioactive form or to zinc (element number 30) or nickel (element number 28). Such reactions were already familiar to nuclear scientists.
What Hahn and Strassmann had seen—and what they had failed to recognize—was a much more dramatic nuclear change. An atom of uranium (element number 92), when struck by a neutron, broke into two much smaller elements such as krypton (element number 36) and barium (element number 56). The reaction was given the name nuclear fission because of its similarity to the process by which a cell breaks into two parts during the process of cellular fission.
Putting nuclear fission to work
In every nuclear fission, three kinds of products are formed. The first product consists of the smaller nuclei produced during fission. These nuclei, like krypton and barium in the example mentioned above, are called fission products. Fission products are of interest for many reasons, one of which is that they are always radioactive. That is, any time a fission reaction takes place, radioactive materials are formed as by-products of the reaction.
Words to Know
Chain reaction: A reaction in which a substance needed to initiate a reaction is also produced as the result of that reaction.
Fission products: The isotopes formed as the result of a nuclear fission reaction.
Fission weapon: A bomb or other type of military weapon whose power is derived from a nuclear fission reaction.
Isotopes: Two or more forms of an element that have the same chemical properties but that differ in mass because of differences in the number of neutrons in their nuclei.
Manhattan Project: A research project of the United States government created to develop and produce the world's first atomic bomb.
Mass: A measure of the amount of matter in a body.
Neutron: A subatomic particle with a mass about equal to that of a hydrogen atom but with no electric charge.
Nuclear reactor: Any device for controlling the release of nuclear power so that it can be used for constructive purposes.
Radioactivity: The property possessed by some elements of spontaneously emitting energy in the form of particles or waves by disintegration of their atomic nuclei.
Radioactive isotope: An isotope that spontaneously breaks down into another isotope with the release of some form of radiation.
Subatomic particle: Basic unit of matter and energy (proton, neutron, electron, neutrino, and positron) smaller than an atom.
The second product of a fission reaction is energy. A tiny amount of matter in the original uranium atom is changed into energy. In the early 1900s, German-born American physicist Albert Einstein (1879–1955) had showed how matter and energy can be considered two forms of the same phenomenon. The mathematical equation that represents this relationship, E = mc 2 , has become one of the most famous scientific formulas in the world. The formula says that the amount of energy (E) that can be obtained from a certain amount of matter (m) can be found by multiplying that amount of matter by the square of the speed of light (c 2 ). The square of the speed of light is a very large number, equal to about 9 × 10 20 meters per second, or 900,000,000,000,000,000,000 meters per second. Thus, if even a very small amount of matter is converted to energy, the amount of energy obtained is very large. It is this availability of huge amounts of energy that originally made the fission reaction so interesting to both scientists and nonscientists.
The third product formed in any fission reaction is neutrons. The significance of this point can be seen if you recall that a fission reaction is initiated when a neutron strikes a uranium nucleus or other large nucleus. Thus, the particle needed to originate a fission reaction is also produced as a result of the reaction.
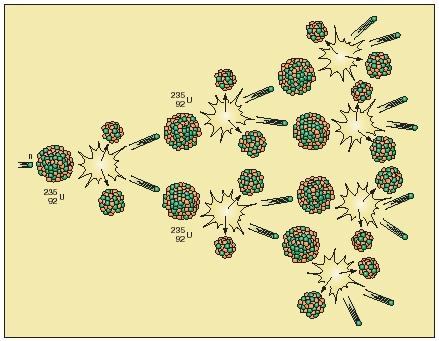
Chain reactions. Imagine a chunk of uranium metal consisting of trillions upon trillions of uranium atoms. Then imagine that a single neutron is fired into the chunk of uranium, as shown in the accompanying figure of a nuclear chain reaction. If that neutron strikes a uranium nucleus, it can cause a fission reaction in which two fission products and two neutrons are formed. Each of these two neutrons, in turn, has the potential for causing the fission of two other uranium nuclei. Two neutrons produced in each of those two reactions can then cause fission in four uranium nuclei. And so on.
In actual practice, this series of reactions, called a chain reaction, takes place very rapidly. Millions of fission reactions can occur in much less than a second. Since energy is produced during each reaction, the total amount of energy produced throughout the whole chunk of uranium metal is very large indeed.
The first atomic bomb
Perhaps you can see why some scientists immediately saw fission as a way of making very powerful bombs. All you have to do is to find a large enough chunk of uranium metal, bombard the uranium with neutrons, and get out of the way. Fission reactions occur trillions of times over again in a short period of time, huge amounts of energy are released, and the uranium blows apart, destroying everything in its path. Pictures of actual atomic bomb blasts vividly illustrate the power of fission reactions.
But the pathway from the Hahn/Strassmann/Meitner/Frisch discovery to an actual bomb was a long and difficult one. A great many technical problems had to be solved in order to produce a bomb that worked on the principle of nuclear fission. One of the most difficult of those problems involved the separation of uranium-238 from uranium-235.
Naturally occurring uranium consists of two isotopes: uranium-238 and uranium-235. Isotopes are two forms of the same element that have the same chemical properties but different masses. The difference between these two isotopes of uranium is that uranium-235 nuclei will undergo nuclear fission, but those of uranium-238 will not. That problem is compounded by the fact that uranium-238 is much more abundant in nature than is uranium-235. For every 1,000 atoms of uranium found in Earth's crust, 993 are atoms of uranium-238 and only 7 are atoms of uranium-235. One of the biggest problems in making fission weapons a reality, then, was finding a way to separate uranium-235 (which could be used to make bombs) from uranium-238 (which could not, and thus just got in the way).
The Manhattan Project. A year into World War II (1939–45), a number of scientists had come to the conclusion that the United States would have to try building a fission bomb. They believed that Nazi Germany would soon be able to do so, and the free world could not survive unless it, too, developed fission weapons technology.
Thus, in 1942, President Franklin D. Roosevelt authorized the creation of one of the largest and most secret research operations ever devised. The project was given the code name Manhattan Engineering District, and its task was to build the world's first fission (atomic) bomb. That story is a long and fascinating one, a testimony to the technological miracles that can be produced under the pressures of war. The project reached its goal on July 16, 1945, in a remote part of the New Mexico desert, where the first atomic bomb was tested. Less than a month later, the first fission bomb was actually used in war. It was dropped on the Japanese city of Hiroshima, destroying the city and killing over 80,000 people. Three days later, a second bomb was dropped on Nagasaki, with similar results. For all the horror they caused, the bombs seemed to have achieved their objective. The Japanese leaders appealed for peace only three days after the Nagasaki event. (Critics, however, charge that the end of the war was in sight and that the Japanese would have surrendered without the use of a devastating nuclear weapon.)
Nuclear fission in peacetime
The world first learned about the power of nuclear fission in the form of terribly destructive weapons, the atomic bombs. But scientists had long known that the same energy released in a nuclear weapon could be harnessed for peacetime uses. The task is considerably more difficult, however. In a nuclear weapon, a chain reaction is initiated—energy is produced and released directly to the environment. In a nuclear power reactor, however, some means must be used to control the energy produced in the chain reaction.
The control of nuclear fission energy was actually achieved before the production of the first atomic bomb. In 1942, a Manhattan Project research team under the direction of Italian physicist Enrico Fermi (1901–1954) designed and built the first nuclear reactor. A nuclear reactor is a device for obtaining the controlled release of nuclear energy. The reactor had actually been built as a research instrument to learn more about nuclear fission (as a step in building the atomic bomb).
After the war, the principles of Fermi's nuclear reactor were used to construct the world's first nuclear power plants. These plants use the
energy released by nuclear fission to heat water in boilers. The steam that is produced is then used to operate turbines and electrical generators. The first of these nuclear power plants was constructed in Shippingport, Pennsylvania, in 1957. In the following three decades, over 100 more nuclear power plants were built in every part of the United States, and at least as many more were constructed throughout the world.
By the dawn of the 1990s, however, progress in nuclear power production had essentially come to a stop in the United States. Questions about the safety of nuclear power plants had not been answered to the satisfaction of most Americans, and, as a result, no new nuclear plants have been built in the United States since the mid-1980s.
Despite these concerns, nuclear power plants continue to supply a good portion of the nation's electricity. Since 1976, nuclear electrical generation has more than tripled. At the beginning of the twenty-first century, 104 commercial nuclear power reactors in 31 states accounted for about 22 percent of the total electricity generated in the country. Combined, coal and nuclear sources produce 78 percent of the nation's electricity.
[ See also Nuclear fusion ; Nuclear power ; Nuclear weapons ]
ThanX!!!
Thanks alot
By Gobi - Laurier Student
Thanks again.