pH
The most common method of indicating the acidity of a solution is by stating its pH. The term pH refers to a mathematical system developed by Danish chemist Søren Sørenson (1868–1939) around 1909. Sørenson originally suggested the term pH as an abbreviation for potential (or power) of hydrogen.
Acids and bases were first defined by Swedish chemist Svante Arrhenius (1859–1927). Arrhenius proposed that acids be defined as chemicals that produce positively charged hydrogen ions, H + , in water. By comparison, he suggested that bases are compounds that produce negatively charged hydroxide ions, OH − , in water.
The pH of a solution is determined by the concentration of hydrogen ions present—that is, by its acidity. The more hydrogen ions present (the more acidic the solution), the lower the pH. The fewer hydrogen ions present (the less acidic the solution), the higher the pH. The pH scale runs from 0 to 14. A pH value of 7 (in the middle of that range) represents a solution that is neither acidic nor basic.
Strong acids have very low pHs (battery acid has a pH of 0). Strong bases have very high pHs (sodium hydroxide, commonly known as lye, has a pH of 14). Lemon juice has a pH of 2; vinegar of 2.5; coffee of 5; distilled water of 7; borax of 9; and household ammonia of 11.
pH indicators
One way of finding the pH of a solution is with a pH meter, a mechanical device that gives very precise readings. One can easily place the probe of a pH meter into a solution and read the pH of the solution on the meter dial.
Table 1.
Some Common Solutions and Their pH

| Substance | Approximate pH |
| Battery acid (sulfuric acid) | 0 |
| Lemon juice | 2 |
| Vinegar | 2.5 |
| Coffee | 5 |
| Distilled water | 7 |
| Borax | 9 |
| Household ammonia solution | 11 |
| Lye (sodium hydroxide) | 14 |
Table 2.
Some Common Indicators, the pH Range in Which They Change Color, and
Their Color Changes
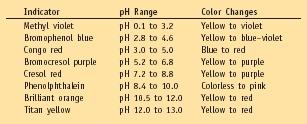
| Indicator | pH Range | Color Changes |
| Methyl violet | pH 0.1 to 3.2 | Yellow to violet |
| Bromophenol blue | pH 2.8 to 4.6 | Yellow to blue–violet |
| Congo red | pH 3.0 to 5.0 | Blue to red |
| Bromocresol purple | pH 5.2 to 6.8 | Yellow to purple |
| Cresol red | pH 7.2 to 8.8 | Yellow to purple |
| Phenolphthalein | pH 8.4 to 10.0 | Colorless to pink |
| Brilliant orange | pH 10.5 to 12.0 | Yellow to red |
| Titan yellow | pH 12.0 to 13.0 | Yellow to red |
A much older method for estimating the pH of a solution is the use of an indicator. A pH indicator is a material that changes color in solutions of different pH. One of the most common of all indicators is litmus. Litmus is a chemical obtained from lichens. In the presence of a base, litmus is blue; in the presence of an acid, it is red.
Many indicators are extracted from plants. For example, you can make a reasonably good indicator just by boiling red cabbage and extracting the colored material produced. That material, like litmus, changes color in the presence of acids and bases. A number of indicators are synthetic products made just for testing the pH of solutions.
[ See also Acids and bases ]