Electrons - How it works
The Electron's Place in the Atom
The atom is discussed in detail elsewhere in this book; here, the particulars of atomic structure will be presented in an abbreviated form, so that the discussion of electrons may proceed. At the center of an atom, the smallest particle of an element, there is a nucleus, which contains protons and neutrons. Protons are positive in charge, while neutrons exert no charge.
Moving around the nucleus are electrons, negatively charged particles whose mass is very small in comparison to the proton or neutron: 1/1836 and 1/1839 the mass of the proton and neutron respectively. The mass of an electron is 9.109389 · 10 −33 g. Compare the number 9 to the number 1,000,000,000,000,000,000,000,000,000,000,000,
and this gives some idea of the ratio between an electron's mass and a gram. The large number—1 followed by 33 zeroes—is many trillions of times longer than the age of the universe in seconds.
IONS AND CHEMICAL BONDS.
Electrons, though very small, are exceedingly powerful, and they are critical to both physical and chemical processes. The negative charge of an electron, designated by the symbol 1− or −1, is enough to counteract the positive charge (1+ or +1) of a proton, even though the proton has much greater mass. As a result, atoms (because they possess equal numbers of protons and electrons) have an electric charge of zero.
Sometimes an atom will release an electron, or a released electron will work its way into the structure of another atom. In either case, an atom that formerly had no electric charge acquires one, becoming an ion. In the first of the instances described, the atom that has lost an electron or electrons becomes a positively charged ion, or cation. In the second instance, an atom that gains an electron or electrons becomes a negatively charged ion, or anion.
One of the first forms of chemical bonding discovered was ionic bonding, in which electrons clearly play a role. In fact, electrons are critical to virtually all forms of chemical bonding, which relates to the interactions between electrons of different atoms.
MOVEMENT OF THE ELECTRON ABOUT THE NUCLEUS.
If the size of the nucleus were compared to a grape, the edge of the atom itself would form a radius of about a mile. Because it is much smaller than the nucleus,
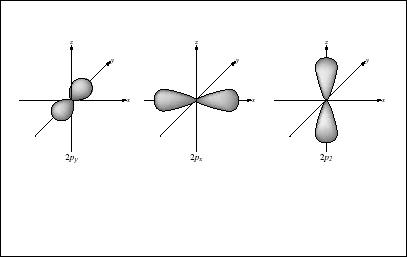
The electron does not move around the nucleus as a planet orbits the Sun—a model of electron behavior that was once accepted, but which has since been overturned. On the other hand, as we shall see, the electron does not simply "go where it pleases": it acts in accordance with complex patterns described by the quantum theory of physics. Indeed, to some extent the behavior of the electron is so apparently erratic that the word "pattern" seems hardly to describe it. However, to understand the electron clearly, one has to set aside all ideas about how objects behave in the physical world.
Emerging Models of Electron Behavior
The idea that matter is composed of atoms originated in ancient Greece, but did not take hold until early in the nineteenth century, with the atomic theory of English chemist John Dalton (1766-1844). In the years that followed, numerous figures—among them Russian chemist Dmitri Mendeleev (1834-1907), father of the periodic table of elements—contributed to the emerging understanding of the atomic model.
Yet Mendeleev, despite his awareness that the mass of an atom differentiated one element from another, had no concept of subatomic particles. No one did: until very late in the nineteenth century, the atom might as well have been a hard-shelled ball of matter, for all that scientists understood about its internal structure. The electron was the first subatomic particle discovered, in 1897—nearly a century after the scientific beginning of atomic theory. Yet the first hints regarding its existence had begun to appear some 60 years before.
DISCOVERY OF THE ELECTRON.
In 1838, British physicist and chemist Michael Faraday (1791-1867) was working with a set of electrodes—metal plates used to emit or collect electric charge—which he had placed at either end of an evacuated glass tube. (In other words, most of the air and other matter had been removed from the tube.) He applied a charge of several thousand volts between the electrodes, and discovered that an electric current flowed between them.
This seemed to suggest the existence of particles carrying an electric charge, and four decades later, English physicist William Crookes (1832-1919) expanded on these findings with his experiments using an apparatus that came to be known as a Crookes tube. As with Faraday's device, the Crookes tube used an evacuated glass tube encased between two electrodes—a cathode at the negatively charged end, and an anode at the positively charged end. A wire led outside the bulb to an electric source, and when electricity was applied to the electrodes, the cathodes emitted rays. Crookes concluded that the cathode rays were particles with a negative electric charge that came from the metal in the cathode plate.
English physicist J. J. Thomson (1856-1940) hypothesized that the negatively charged particles Crookes had observed were being emitted by atoms, and in 1897 he gave a name to these particles: electrons. The discovery of the electron raised questions concerning its place in the atom: obviously, there had to be a counterbalancing positive charge, and if so, from whence did it come?
FROM PLUM PUDDINGS TO PLANETS.
Around the beginning of the twentieth century, the prevailing explanation of atomic structure was the "plum pudding model," which depicted electrons as floating like raisins in a "pudding" of positive charges. This was overturned by the discovery of the nucleus, and, subsequently, of the proton and neutron it contained.
In 1911, the great Danish physicist Niels Bohr (1885-1962) studied hydrogen atoms, and concluded that electrons move around the nucleus in much the same way that planets move around the Sun. This worked well when describing the behavior of hydrogen, which, in its simplest form—the isotope protium—has only one electron and one proton, without any neutrons. The model did not work as well when applied to other elements, however, and within less than two decades Bohr's planetary model was overturned.
As it turns out, the paths of an electron's movement around the nucleus are nothing like that of a planet's orbit—except inasmuch as both models describe a relatively small object moving around a relatively large one. The reality is much more complex, and to comprehend the secret of the electron's apparently random behavior is truly a mind-expanding experience. Yet Bohr is still considered among the greatest scientists of the twentieth century: it was he, after all, who first explained the quantum behavior of electrons, examined below.
Comment about this article, ask questions, or add new information about this topic: