Greenhouse effect
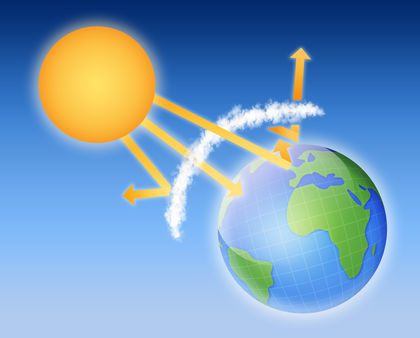
The greenhouse effect is a natural phenomenon that is responsible for the relatively high temperature maintained on Earth's surface and in its atmosphere. The name comes from the process by which greenhouses are thought to collect and hold heat.
The greenhouse mechanism
A greenhouse is a building in which plants are grown and kept. It usually consists of a large expanse of window glass facing in a generally southerly direction. Sunlight that strikes the windows of the greenhouse passes through those windows and strikes the ground inside the greenhouse. This process is possible because glass is transparent to sunlight, that is, it allows sunlight to pass through.
Sunlight that strikes the ground inside a greenhouse either may be reflected or absorbed by the ground. Sunlight that is absorbed by the ground may later be re-emitted in the form of heat waves. When it bounces back towards the windows of the greenhouse, it is not able to pass back through the windows. In either instance, the sunlight undergoes a change in form once it enters the through the windows. The windows are not transparent, but are opaque, to the reflected and reradiated energy. The energy trapped inside the greenhouse is then used to raise the temperature inside the greenhouse. It is this effect that makes it possible for a greenhouse to stay warm even though the outside temperature is quite cold.
The greenhouse effect in Earth's atmosphere
An effect similar to the one just described also occurs in Earth's atmosphere. The atmosphere does not have a glass window, of course, although the gases that make up the atmosphere act something like a window.
Words to Know
Atmospheric window: A range in the wavelength of radiations, from about 350 to 750 nanometers, that can pass through Earth's atmosphere without being absorbed.
Equilibrium: A process in which the rates at which various changes take place balance each other, resulting in no overall change.
Frequency: The number of waves that pass a given point every second.
Infrared radiation: Another name for heat; a form of radiation with wavelengths in the range from about 700 nanometers to 1 millimeter.
Nanometer: One-billionth of a meter.
Radiation: Energy emitted in the form of waves or particles.
Visible light: A form of radiation with wavelengths in the range from about 400 to about 750 nanometers.
Wavelength: The distance between any two successive crests or troughs in a wave.
Imagine a burst of solar energy reaching the outer edges of Earth's atmosphere. That solar energy consists of many different kinds of radiation, such as visible light, ultraviolet radiation, infrared radiation, X rays, gamma rays, radio waves, and microwaves. These forms of radiation are different from each other in that they all travel with different wavelengths and different frequencies. (The wavelength of a wave is the distance between any two successive crests or troughs [pronounced trawfs] in the wave. The frequency of the radiation is the number of waves that pass a given point every second.) For example, X rays have very short wavelengths and very large frequencies. In contrast, radio waves have very long wavelengths and very small frequencies. The important point, however, is that all forms of radiation, whatever their wavelength and frequency, travel together in a burst of solar energy.
The energy that reaches Earth's atmosphere can experience one of three fates, depending on the kind of radiation and the kind of gases present in the atmosphere. First, about one-third of all the solar energy that reaches Earth's atmosphere is reflected back into space. As far as Earth is concerned, that energy is simply lost to space.
Another one-third of the solar energy is absorbed by gases in Earth's atmosphere. Various gases absorb various types of radiation. Oxygen and ozone, for example, tend to absorb radiation with short wavelengths, such as ultraviolet light. In contrast, carbon dioxide and water absorb radiation with longer wavelengths, such as infrared radiation. When these gases absorb various types of radiation, they convert the energy of sunlight into heat. This phenomenon accounts for some of the heat stored in Earth's atmosphere.
Yet another one-third of the solar energy reaching our atmosphere is able to pass through the atmosphere and strike Earth's surface. This process is similar to the way sunlight is transmitted through windows in a greenhouse. The solar energy that passes through Earth's atmosphere does so because there are very few gases that absorb visible radiation.
Scientists sometimes refer to a "window" in Earth's atmosphere (somewhat similar to a greenhouse window) through which radiation can pass. That atmospheric window is not an object, like a piece of glass, but a range in radiation across which atmospheric gases are transparent. That range is from about 350 to 750 nanometers (a nanometer is one-billionth of a meter). For comparison, the wavelengths of visible light range from about 400 nanometers (for blue light) to 750 nanometers (for red light).
Reflection and absorption. The solar energy that reaches Earth's surface also can experience a number of fates. It can cause ice to melt and water to evaporate; it can be used to convert carbon dioxide and water to carbohydrates in plants (photosynthesis); it can heat parcels of air and water, causing winds, waves, and currents; and it can heat Earth's surface. The last of these fates is the most important. About one-half of all the solar energy that passes through the atmosphere is absorbed by soil, rocks, sand, dirt, and other natural and human-made objects.
All of which is to tell you something you already know. If you put your hand on a patch of dark soil at the end of a sunny day, the soil feels warm. In fact, if you place your hand just above the soil, you can feel heat being given off by the soil. The reason is that objects that are heated by sunlight behave in the same way as the ground in a greenhouse. Those objects give back energy picked up from sunlight, but in a different form. Instead of reradiating the energy in the form of visible light, the objects give the energy back off in the form of heat.
But what happens when heat reradiated from Earth's surface travels back upwards into the atmosphere. Heat is a form of infrared radiation. As pointed out previously, carbon dioxide and water are both good absorbers of infrared radiation. So the very gases that allowed visible light to pass through the atmosphere are now able to absorb (trap) the infrared radiation (heat) reradiated from Earth's surface.
The sum total of all these reactions is that energy from the Sun is captured by both Earth's surface and the gases in the atmosphere. As a result, the planet's annual average temperature is about 55°F (30°C) higher than it would be without an atmosphere.
Human activities and the greenhouse effect
Natural phenomena, such as the greenhouse effect, reach a natural state of equilibrium over many hundreds or thousands of years. (A state of equilibrium is a process in which the rates at which various changes take place balance each other.) Two factors that affect the greenhouse effect are the shape of Earth's orbit and its tilt with regard to the Sun. Both of these factors change slowly over hundreds of thousands of years. When they do, they alter the effects produced by the greenhouse effect. For example, suppose that Earth's orbit changes so that our planet begins to move closer to the Sun. In that case, more solar energy will reach the outer atmosphere and, eventually, the planet's annual average temperature will probably increase.
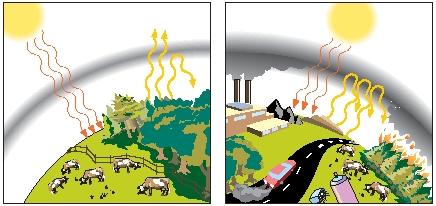
In recent years, scientists have been exploring the possibility that various human activities also may influence the greenhouse effect. The most important of these activities is thought to be the combustion (burning) of fossil fuels, such as coal, oil, and natural gas.
No one needs to be reminded today of the important role of fossil fuels in our society. We use them for heating homes and offices; for powering our cars, trucks, railroads, airplanes, and other forms of transportation; and for operating industrial processes. But the combustion of any fossil fuel always results in the release of carbon dioxide and water into the atmosphere. By some estimates, the release of carbon dioxide into the atmosphere from fossil fuel combustion reached almost 2.5 billion tons (2.3 billion metric tons) in 1999.
The result of this human activity is that Earth's atmosphere contains a higher concentration of carbon dioxide today than it did a century ago. The most reliable scientific data show that the concentration of carbon dioxide in the atmosphere has increased from about 320 parts per million in 1960 to nearly 360 parts per million today.
Effects of increasing concentrations of carbon dioxide
Many scientists are concerned about the increasing levels of carbon dioxide in Earth's atmosphere. With more carbon dioxide in the atmosphere, they say, more heat will be trapped. Earth's annual average temperature will begin to rise. In early 2001, in a striking report released by the Intergovernmental Panel on Climate Change (a United Nationssponsored panel of hundreds of scientists), scientists concluded that if greenhouse emissions are not curtailed, the average global surface temperature could rise by nearly 11°F (6°C) over the next 100 years. The scientists also stated that man-made pollution has "contributed substantially" to global warming and that Earth is likely to get a lot hotter than previously predicted.
Such a rise in temperature could have disastrous effects on the world. One result might be the melting of Earth's ice caps at the North and South Poles, with a resulting increase in the volume of the ocean's water. Were that to happen, many of the world's largest cities—those located along the edge of the oceans—might be flooded. Some experts predict dramatic changes in climate that could turn currently productive croplands into deserts, and deserts into productive agricultural regions. Half of all Alpine glaciers would disappear. Coral reefs would be destroyed, and vulnerable plant and animal species would be pushed to extinction.
In 1997, in Kyoto, Japan, representatives from more than 170 nations met to try to agree to decisive actions to reduce their emissions of
greenhouse gases. A treaty, called the Kyoto Protocol, was drafted at the meeting that proposed that 38 industrial nations cut their greenhouse-gas emissions by 2012 to 5.2 percent below levels in 1990 (the United States is the biggest producer of greenhouse gases, producing about 25 percent of the gases associated with global warming; Japan and Russia are the next biggest producers). More than 150 nations signed the treaty, but no industrialized country ratified it, and the treaty cannot take effect until a substantial number of industrial nations ratify it.
In November 2000, in The Hague, Netherlands, officials from around the world met to write the detailed rules for carrying out the Kyoto Protocol. Unfortunately, after less than two weeks, the talks collapsed in disarray with no deal reached to stop global warming. The main reason for the collapse was the argument between the United States and European countries over ways to clean up Earth's atmosphere. Officials attending the meeting did agree to meet once more to tackle the issue of global warming.
Differences of opinion. As with many environmental issues, experts tend to disagree about one or more aspects of anticipated climate change. Some authorities are not convinced that the addition of carbon dioxide to the atmosphere will have any significant long-term effects on Earth's average annual temperature. Others concede that Earth's temperature may increase, but that the changes predicted are unlikely to occur. They point out that other factors—such as the formation of clouds—might counteract the presence of additional carbon dioxide in the atmosphere. They warn that nations should not act too quickly to reduce the combustion of fossil fuels since that will cause serious economic problems in many parts of the world. They suggest, instead, that we wait for a while to see if greenhouse factors really are beginning to change.
The problem with that suggestion, of course, is that it is possible to wait too long. Suppose that fossil fuel combustion is causing significant changes in the climate. It might take a half century or more to be certain of the relationship between fossil fuel combustion and warmer planetary temperatures. But at that point, it also might be too late to resolve the problem very easily. The carbon dioxide would have been already released to the atmosphere, and climate changes would have already begun to occur. As evidenced by the collapse of climate talks and the failure to ratify the Kyoto Protocol, there is no general consensus among scientists, politicians, businesspeople, and the general public as to what, if anything should be done about the potential for climate change on our planet.
The battle between industry and environmentalists over the issue of global warming continues with no clear vision for the future. In March 2001, U.S. President George W. Bush all but put an end to the possibility that the United States would follow through with the Kyoto Protocol when he said his administration would not seek to curb the emissions of carbon dioxide from power plants. This was a sharp reversal from his position during the presidential campaign in 2000. Ignoring recently published scientific reports, Bush stated that he made his decision "given the incomplete state of scientific knowledge of the causes of, and solutions to, global climate change."
[ See also Carbon cycle ; Carbon dioxide ; Forests ; Ozone ; Pollution ]
Isn't specific heat capacity supposed to have a baring global warming?
help me now please i have a 500 words essay.