OSMOSIS
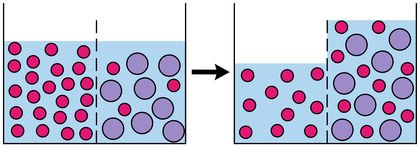
The term osmosis describes the movement of a solvent through a semipermeable membrane from a less concentrated solution to a more concentrated one. Water is sometimes called "the perfect solvent," and living tissue (for example, a human being's cell walls) is the best example of a semipermeable membrane. Osmosis has a number of life-preserving functions: it assists plants in receiving water, it helps in the preservation of fruit and meat, and is even used in kidney dialysis. In addition, osmosis can be reversed to remove salt and other impurities from water.
If you were to insert a hollow tube of a certain diameter into a beaker of water, the water would rise inside the tube and reach the same level as the water outside it. But suppose you sealed the bottom end of the tube with a semipermeable membrane, then half-filled the tube with salt water and again inserted it into the beaker. Over a period of time, the relative levels of the salt water in the tube and the regular water in the beaker would change, with the fresh water gradually rising into the beaker.
This is osmosis at work; however, before investigating the process, it is necessary to understand at least three terms. A solvent is a liquid capable of dissolving or dispersing one or more other substances. A solute is the substance that is dissolved, and a solution is the resulting mixture of solvent and solute. Hence, when you mix a packet of sugar into a cup of hot coffee, the coffee—which is mostly water—acts as a solvent for the sugar, a solute, and the resulting sweetened coffee is a solution. (Indeed, people who need a cup of coffee in the morning might say that it is a "solution" in more ways than one!) The relative amount of solute in the solution determines whether it can be described as more or less concentrated.
This website is fantastic and I applaud the person who put this information together! Truly priceless to have, as an educator!
What is diffusion ? Give me examples of diffusion.
CAHS
Thanks bro